Reports: DNI555900-DNI5: Targeted Size Mesoporous Zeolites for Deep Desulfurization of Petroleum Streams via Adsorption
Ioulia Valla, PhD, University of Connecticut
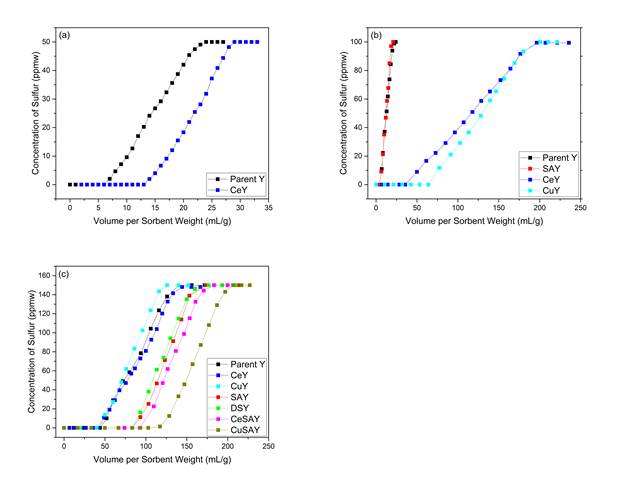
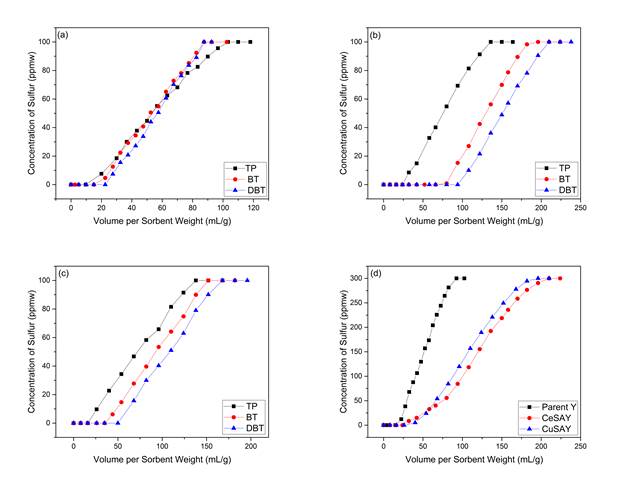
The goal of the study was to investigate the role of metal-exchanged mesoporous Y zeolites for the adsorptive desulfurization of fuels. Our hypothesis was that the introduction of mesoporosity would allow larger molecules such as DiBenzothiophene (DBT), to access the active sites of the micropores via σ-bonding or π-complexation. Τhe novelty of this study is that the combination of both functions of metal-exchanged mesoporous Y zeolites, designated as SAY, can significantly improve the adsorptive desulfurization in a fixed-bed application. Hence, CeSAY and CuSAY zeolites were prepared to test this hypothesis. Figure 1&2 confirm the results from desulfurizing model fuels with CeSAY and CuSAY. Capacity for DBT was significantly increased especially with CuSAY compared to Y. DBT exhibits relatively higher electron density than other sulfur molecules, which makes it highly favorable for adsorption on the active sites. However, the presence of DBT causes steric hindrance due to its high kinetic diameter of ~9Å and inability for other molecules to access the active sites, suggesting that diffusion can be a limiting factor. The mass transfer limitations can be overcome by making the parent Y mesoporous. As pore access becomes possible, the thermodynamic equilibrium constant increases, which results in higher sulfur uptake. For smaller sulfur compounds such as thiophene (TP) and (BT), the relatively smaller kinetic diameters allow them to access the supercage freely and subsequently the active sites without any diffusion limitations. We suggest that the adsorptive desulfurization by metal-exchanged mesoporous Y zeolites is mainly driven by thermodynamics.
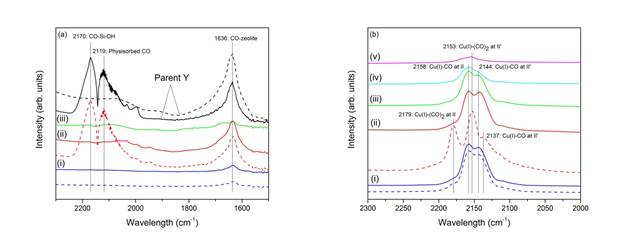
Our results indicate that Cu has a higher capacity than that of Ce in the adsorption of single sulfur compound. Analysis of our FTIR results clarifies and distinguishes the difference on the location of Ce and Cu metals. In Figure 3, chemisorbed CO is absent from the CeY spectrum, whereas strongly-adsorbed CO was observed in the CuY spectrum. CeY occupies obscure locations such as Site I and I’ which are too confined for CO to enter. Ce cations favor the migration into the hidden sites, because they can form higher coordination bonds with 6 oxygen ligands. The pores leading to these sites, however, are relatively small and would not permit the access of CO molecules. Hence, the absence of chemisorbed-CO vibrational peaks on CeY samples. Sites II and II’ occupied by Cu, on the other hand, are very approachable by CO, as well as for the adsorption of TP and other relevant compounds. However, in the mixture of multiple sulfur compounds, Ce cations have shown to be more selective for DBT removal due to strong direct S-M bonds. This could be due to the migration of Ce cations in hidden sites toward the supercage as a result of the tendency to form high energy complexes with thiophenes via the strong σ-bond interaction. These results suggest that the affinity of thiophenic molecules to adsorb on the active sites would depend on the type of metal and its location.
To confirm this adsorption trend, a mixture of model fuel containing all three sulfur compounds were tested on metal-exchanged mesoporous zeolites. Figure 2 shows that that DBT is most strongly adsorbed compared to BT and T, confirming the aforementioned trend. This leads us to conclude that DBT exhibits the highest electron density among the competing molecules and thus, would form the highest energy bond with the active site. Heats of adsorption were also measured to determine the nature and bond strength exhibited between thiophenes and zeolites using the isosteric method. Results showed that ΔHads is the highest for CuY for both BT and DBT compounds, which is up to twice as much compared to the parent Y. The increase in ΔHads shows that Cu metals are responsible for stronger sulfur adsorption, which increases the bond strength between the sorbent and the sorbate. This also indicates that the higher adsorption energy is thermodynamically favored, thus increasing the sorbate uptake at equilibrium on CuY. To confirm the hypothesis, the ΔHads of DBT on CuSAY was measured and the results showed a decrease in energy value. The introduction of mesoporosity may grant access to diffusion into the internal sites, but the trade-off is a reduction of adsorption sites. The design of metal-exchanged mesoporous Y zeolites explores the balance between the embodiment of active metals and mesopores for adsorptive desulfurization of fuels. For instance, overloading of metals can cause formation of oxides that may block active sites. Also, mesoporosity should be introduced carefully to avoid uncontrolled desilication. Our results have shown that most of the crystal structure of modified materials were retained. The impact of regeneration on the lifetime of sorbent will be addressed in our next studies. Overall, good correlation has been demonstrated between theoretical insight and experimental results. It should be pointed out that in this study octane has been used as a model fuel. However, transportation fuels contain a vast number of aromatic hydrocarbons, which might have an inhibiting effect on the adsorption of sulfur compounds. The viability of metal-exchanged mesoporous zeolites in the presence of aromatics will be explored in our next studies.
The ACS/PRF award has greatly enhanced the PI’s career. One PhD student, Kevin X. Lee, is currently working in this project. The last year’s study has resulted in one peer reviewed paper (see below) and multiple presentations including ACS meetings.
Kevin X. Lee and Julia A. Valla “Investigation of metal-exchanged mesoporous Y zeolites for the adsorptive desulfurization of liquid fuels” Applied Catalysis B. Environmental, 2017, (201), 359–369
Figure 1: Breakthrough curves of: a) thiophene, b) Benzothiophene and c) Dibenzothiophene
Figure 2: Breakthrough curves of model fuel on (a) NH4Y, (b) CeSAY, (c) CuSAY and (d) all sorbents
Figure 3: FTIR spectra of CO adsorption on (a) CeY and (b) CuY at the following conditions: (i) 0.25% CO, (ii) 5% CO, (iii) outgassed at 80 ◦C, (iv) outgassed at 150 ◦C, and (v) outgassed at 180 ◦C. The dotted lines (- - -) represent the spectra after outgassing at room temperature.]