Reports: DNI1055884-DNI10: Rational Synthesis and Characterization of Non-Layered Two-Dimensional Crystals
Guihua Yu, PhD, University of Texas at Austin
[Scientific Goals]: This ACS-PRF research project aims to develop novel self-assembled two-dimensional (2D) crystals and related nanomaterials, and to better understand the structure-property relationship of these 2D nanostructured solids for applications in energy-related devices.
[Progress]: During the first year, significant progress has been made towards the above goal. We recently developed several novel 2D self-assembled oxides and phosphates based nanomaterials such as ternary oxide such as LiNi1/3Co1/3Mn1/3O2 (NCM), and VOPO4 ultrathin nanosheets materials by microwave-assisted solvothermal or chemical exfoliation methods through better understanding of how solvent molecules interact with precursor molecules and/or in-situ self-assembled synthetic approach for controlled growth of complex nanoarchitectures. We also carefully characterized their chemical structures and physical properties.
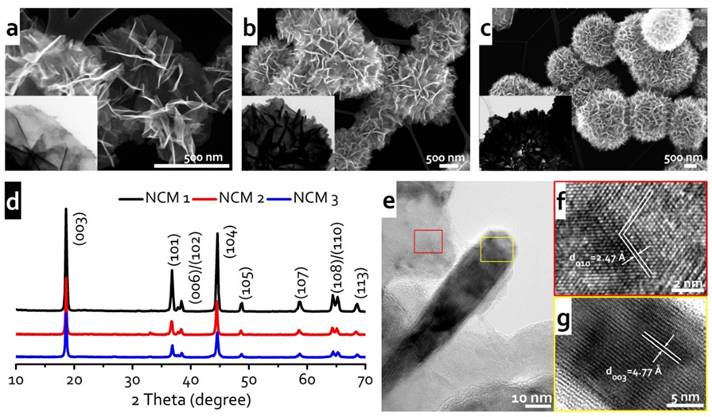
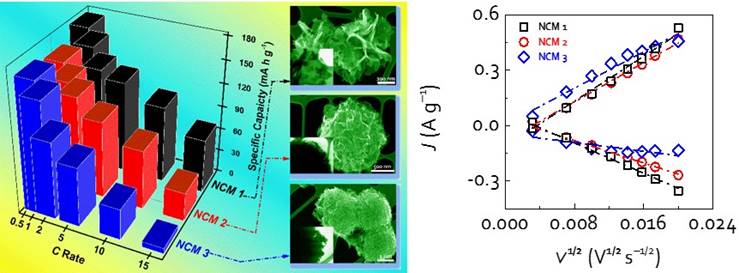
For example, Figure 1 shows our efforts in controlled synthesis of hierarchical assembled LiNi1/3Co1/3Mn1/3O2 (NCM) nanosheets with different surface structures and packing degrees via a hydrothermal method. By tuning the synthetic concentration of precursor chemicals, different surface structures and packing degrees could be readily achieved. Given that different self-assembled surface structures resulted in various ratio of exposed {010} facets, they exhibited different Li ion transport kinetics. As a result, NCM 1 nanomaterials with more exposed {010} facets showed superior rate capability, while NCM 3 samples with much fewer exposed {010} facets and a dense packing showed inferior rate capability (Fig. 2). This systematic study may provide an interesting material model for detailed investigation of the fundamental relationship between the structures and properties, as well as the fabrication of materials with well-controlled size, shape and surface structures.
Fig. 1. Structural characterization of NCM nanomaterials with different surface structures. (a-c) STEM images of the NCM 1, NCM 2, and NCM 3 nanomaterials. (d) XRD patterns. (e) TEM images of as-assembled NCM 1 cathodes showing the representative surface structure and their HRTEM images (f, g).
Fig. 2. Novel nanoarchitectured LiNi1/3Co1/3Mn1/3O2 cathodes composed of different self-assembled single-crystalline nanosheet structures exhibit tunable high rate capability.
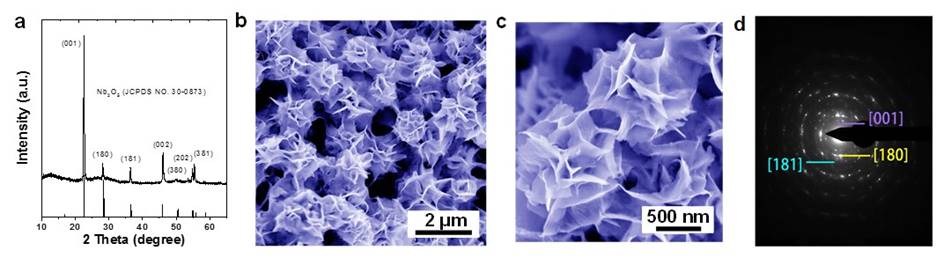
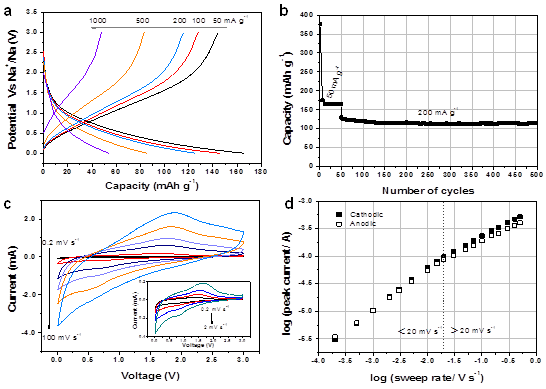
Another recent study is on rational synthesis of self-assembled Nb2O5 nanosheets through a hydrothermal method (Fig. 3). The self-assembled Nb2O5 flower-like nanosheets were achieved by controlling the reaction kinetics and the growth mechanism was simultaneously revealed (from 0D particles to 1D to 2D). As pH changes systematically, various Nb2O5 morphologies could be obtained. Furthermore, the obtained Nb2O5 nanosheets anodes with favorable architectures were studied for sodium ion storage property (Fig. 4), and we found that self-assembled flower-like nanosheets electrode yield outstanding high-rate capability (can deliver high capacity for fast charge/discharge rates).
Fig. 3. Morphology and structure of the in-situ self-assembled Nb2O5 nanosheets. (a) XRD pattern , (b, c) FESEM images of the Nb2O5 nanosheets self-assemble into flower-like structure, (d) SAED patterns of Nb2O5 nanosheets.
Fig. 4. Electrochemical characteristics of the Nb2O5 nanosheets based electrodes. (a) charge-discharge profiles, (b) Cycling stability, (c) CV curves at various scan rates, (d) analysis of charge storage mechanism.
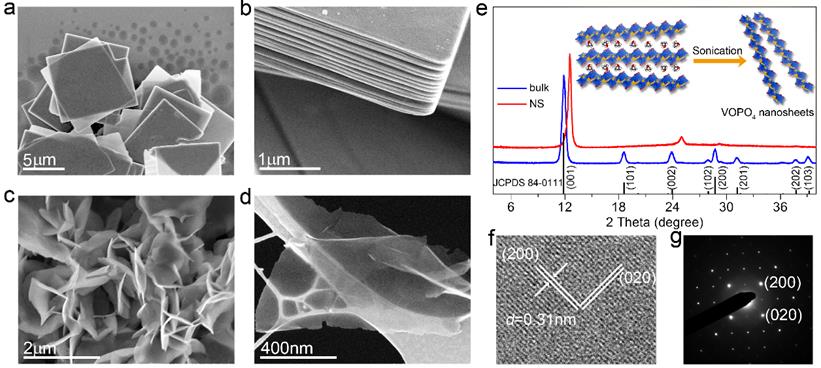
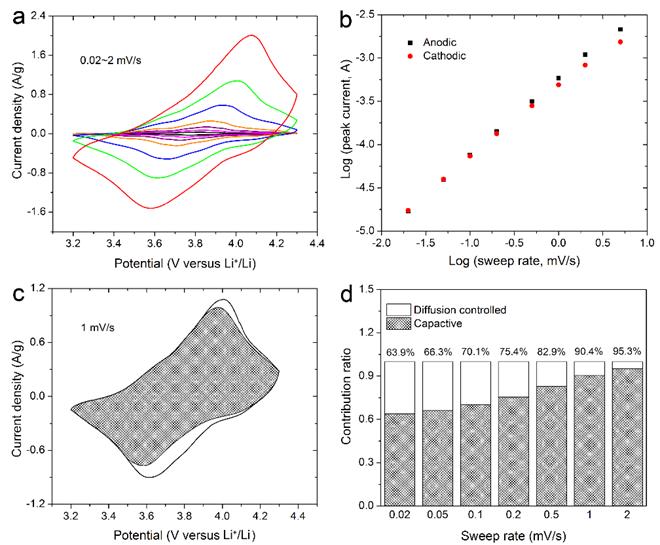
In addition to oxides, we also extended our research efforts into another material, vanadyl phostate (VOPO4). We used ultrathin VOPO4 nanosheets (Fig. 5) as a baisc material platform to a new type of charge storage mechanism for alkali-ion such as Li+, Na+, called intercalation pseudocapacitance, in which ions intercalate into the tunnels or layers of a redox-active material accompanied by a faradaic charge-transfer with no crystallographic phase change. This type of charge storage mechanism holds promise in enabling ultrahigh-rate electrochemical energy storage. The VOPO4 nanosheets obtained through controlled ultrasonication in 2-propanol, exhibit impressive high rate capability (Fig. 6). We experimentally analyzed the contribution of both capacitive-process and diffusion-controlled process, and determined charge storage is primarily resulted from alkali-ion intercalation pseudocapacitance.
Fig. 5. Morphological and structural characterization of VOPO4 samples. (a-b) SEM images of as-synthesized bulk VOPO4 materials. (c-d) SEM image of exfoliated VOPO4 nanosheets. (e) XRD patterns of bulk and exfoliated VOPO4 nanosheets. (f, g) HRTEM image and associated SAED pattern of VOPO4 nanosheets.
Fig. 6. Kinetics analysis of the electrochemical behavior towards alkali ion (Li+ and Na+) for the VOPO4 nanosheets electrode. a) CV curves at various scan rates. b) Determination of the b-value. c) Separation of the capacitive (shaded) and diffusion currents. d) Contribution ratio of the capacitive and diffusion-controlled charge.
[Significance and Impact]: To summarize, we have made significant progress towards both controlled synthesis of some novel 2D crystals based nanomaterials, and better understanding of electrochemical properties of these self-assembled 2D nanostructured materials for energy storage applications. Current research progress laid foundation for the future work on developing bottom-up controlled synthesis of 2D crystals for various types of materials to ultimately enable ‘new materials by design’ for emerging energy technologies. The graduate students working on this project have also gained important training in synthesis and characterization of nanostructured solids, and are making very positive progress towards their PhD degree.