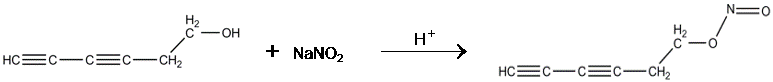Reports: UR453036-UR4: FTIR Analysis of the Radical and Molecular Products of Thermal Decomposition of Aldehydes and Nitrite Esters
Laura R. McCunn, PhD, Marshall University
Technical Progress: Pyrolysis of Branched-Chain Aldehydes
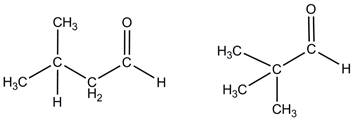
The pyrolysis of branched-alkyl chain aldehydes was studied in a pulsed hyperthermal nozzle. The goal of these experiments was to identify, via matrix-isolation FTIR, the products of gas-phase pyrolysis of pivaldehyde and isovaleraldehyde. (Figure 1) This information will ultimately lead to a better understanding of the pyrolysis mechanism. The results will be compared to the pyrolysis products of small, unbranched aldehydes that are described in the literature: acetaldehyde, propionaldehyde, and butyraldehyde.
Figure 1. Isovaleraldehyde (left) and pivaldehyde (right)
In the third year of PRF support, analysis of the FTIR spectra collected following the 600-1200 ºC pyrolysis of isovaleraldehyde and pivaldehyde was completed. Products identified include: carbon monoxide, vinyl alcohol, ketenes, and several unsaturated hydrocarbons. We have confirmed that isovaleraldehyde produces 3-methyl-1-butyne, likely a product of a tautomerism and water elimination reaction that has been observed in unbranched aldehydes. Pivaldehyde does not produce the butyne, or water, because of the t-butyl structure it contains. Overall, pivaldehyde produces a smaller number of products than isovaleraldehyde under similar conditions because of its highly branched structure.
Technical Progress: Isolation of the
1,3-Pentadiynyl Radical
Figure 2. Attempted synthetic route to a
pyrolytic precursor of the 1,3-pentadiynyl radical
Impact on Undergraduate Students Three students were directly supported by PRF funds in the
summer of 2015. Sarah Cole is a senior majoring in chemistry. John Sowards is
a junior who recently switched from a chemistry major to a secondary education
major, focusing on chemical education. Martha Ellis is a sophomore chemistry
major whose summer support was provided by both the PRF grant and a
departmental endowment funded by alumni. All three students collaborated in
the collection of matrix-isolation FTIR spectra for the projects described here,
but each one was designated the lead student for their own project. These
students are continuing their research in the 2016-2017 academic year.
The benefit to the involved students far exceeds their
summer employment. The students participated in the Department of Chemistry’s
summer research program, which included approximately 20 undergraduate
students, local high school students and teachers working in Marshall chemistry
faculty labs. Participants gave short proposal talks at a kickoff luncheon,
toured the West Virginia State Police Forensics Laboratory, and engaged in
social activities with professors. The summer experience culminated in a
formal research symposium featuring 10-15 minute oral presentations. Because
of her research experience, the Department of Chemistry gave Sarah Cole the
opportunity to attend the American Chemical Society Fall National Meeting in
Philadelphia in order to network and enhance her technical skills. She is
currently writing an abstract to present her work at the Spring National
Meeting in San Francisco. It is anticipated that every student supported by
PRF will have an opportunity to attend or present research at a conference,
which will greatly enrich their professional development.
Impact on Career of the PI Research support from PRF has enabled the PI to retain
undergraduate researchers for multiple years of research. This allows students
to gain independence in their daily experiments and gives them time to see
projects through to the end. The PI has delivered two oral presentations based
on PRF-supported projects at the 2015 and 2016 meetings of the International
Symposium of Molecular Spectroscopy. PRF support has thus far resulted in one
publication, another under review at the time of this report, and the potential
for two more manuscript submissions in the coming year. In the past three
years, the PI’s laboratory has experienced a dramatic increase in productivity
due to PRF support.