Reports: DNI554905-DNI5: Controlling Polymerization Reactions at the Nanoscale
Ke Zhang, PhD, Northeastern University
Noble metal nanoparticles (NPs) resonantly absorb electromagnetic energy via plasmons and convert it into thermal energy, which leads to local heating near the NP surface. This photothermal effect of NPs has been widely applied in hyperthermal therapy, genetic engineering, drug delivery, and nanolithography. The objective of this project is to investigate how Atom Transfer Radical Polymerization (ATRP) reactions can be controlled by photothermal effect near gold nanoparticles (AuNPs) surface. In order to accomplish this goal, we functionalize gold nanoparticles with ATRP initiators, and apply light to induce the photothermal effect. This project was divided into two phases. The first phase is to find appropriate solvent system, which can stabilize AuNPs during polymerization. We found that ATRP catalyst, e.g. Cu(I) or Cu(II) either free or complexed, in aqueous solution would cause the aggregation of citrate-stabilized AuNPs. Therefore, it is critical to transfer AuNPs into organic solvent, such as tetrahydrofuran (THF), dimethylformamide (DMF), or isopropanol, where the AuNPs remain stable in the reaction mixture. The second phase involves studying the reaction conditions to promote photothermal ATRP while suppressing bulk ATRP. Considerations include the choice of a proper monomer, appropriate light intensities that could induce ATRP but not extremely high temperatures that can damage the thiol-gold interactions, and an oxygen-free solvent system (oxygen is a radical quencher and can also oxidize the thiol-gold bond).
Results.
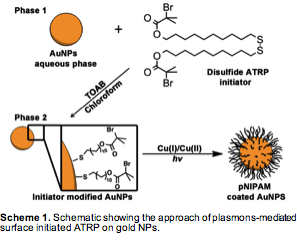
I. Phase transfer of AuNPs. Our initial efforts focused on synthesizing AuNPs in organic solved per literature reports. However, the uniformity of AuNPs produced in organic phase has unacceptable polydispersity compared with those made with the Frens-Turkevich method (citrate stabilized AuNPs in aqueous phase). Indeed, particle geometry plays an important role in the utilization of noble metal particles' photothermal effect. Therefore, we developed a new strategy to functionalize citrate-stabilized AuNPs with ATRP initiators. The experiments start with the reduction of HAuCl4 with sodium citrate, which resulted 12 nm gold nanoparticles in aqueous phase. We then utilized a phase transfer catalyst, tetraoctylammonium bromide (TOAB), to transfer the AuNPs from the aqueous phase to chloroform solution containing an ATRP initiator, bis[2-(2-bromoisobutyryloxy)undecyl] disulfide.
Figure 1a showed quantitative transfer into chloroform. Excess ATRP initiators were removed by 3x centrifugation-resuspension. We used transmission electron microscopy (TEM) to characterize the ATRP initiator modified AuNPs, and found that the transfer process didn't jeopardize the uniformity or aggregation state of AuNPs (Figure 1b). The resulting ATRP initiator-modified gold NPs are soluble in common organic solvents, e.g. THF, DMF, for several months.
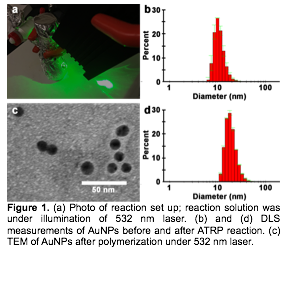
II. ATRP on AuNPs surface. We found that light alone is able to cause the aggregation of ATRP initiator-functionalized AuNPs, a phenomenon that we attribute to the oxidation of the thiol-gold bond under high temperatures. To minimize the thiol-gold bond dissociation, we chose n-isopropylacrylamide (NIPAM) as our model monomer, which can be polymerized at relatively low temperatures (40°C). Initially, we used white light (produced with a 100W halogen lamp) coupled with a low-pass filter as the light source, and optical fibers as a conduit to deliver light to a glass reaction vessel containing the reaction mixture, which is placed in ice water. We found that polymerization occurs both in the bulk and on the AuNP surface. It is likely that the light source is causing unintended bulk heating. Therefore, we next acquired a 532 nm laser (20 mW-1W tunable) as the light source, due to the fact that 13 nm spherical AuNPs have strong surface plasmon resonance at this wavelength. Both DLS measurements and TEM images showed the increased diameter of AuNPs, which indicate that pNIPAM coated AuNPs have been achieved.
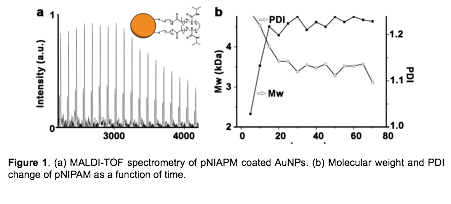
In order to have a better understanding of the polymerization kinetics, we characterized the polymerization process by Matrix Assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF) mass spectrometry. The molecular weight of polymers attached to gold nanoparticles was measured every 5 minutes (Figure 3a). The molecular weight increase rapidly initially, but stopped growing at ~15 minutes, which suggests that there exists a range of photothermal effect from the surface. Beyond this range, the thermal energy wound not be enough to sustain the polymerization of NIPAM. The first 20 minutes of polymerization of NIPAM exhibited first-order kinetics, which is consistent with ATRP characteristics. We next tested other monomers that require relatively higher temperatures for ATRP reactions, including a series of acrylates which usually polymerize around 60°C, such as t-butyl acrylate, n-butyl acrylate, (hydroxyethyl) methacrylate, and methyl methacrylate. Unfortunately, we did not observe any polymerization for these monomers. It appears that photothermal effect from 13 nm spherical AuNPs is not sufficient to initiate polymerization of monomers that require greater energy.
Future plan and conclusion. Our next step is to conduct photothermal ATRP on anisotropic nanoparticles, such as gold nanorods and prisms. Anisotropic AuNPs exhibit a much stronger photothermal effect than spherical AuNPs under near-infrared light. Further, the polymerization can be regiospecific, i.e. only at specific locations of the nanoparticle. This is because anisotropic NPs do not exhibit homogeneous near-field intensity enhancements. Highly curved regions, such as the termini of rods and the tips of prisms, often exhibit the greatest level of field localization.
In conclusion, we report a new concept of surface initiated ATRP polymerization using a plasmon mediated process. Our method can be used to supplement the 'graft-onto' methodology for functionalizing NPs. In the future, we plan to extend our method to establish the much-needed capability to functionalize NPs in a regiospecific fashion for colloid crystal engineering.