Reports: ND153705-ND1: Organocatalytic Carbonyl-Olefin Metathesis and Olefin Metathesis
Tristan H. Lambert, PhD, Columbia University
The objective of this program is to develop a highly efficient and broadly applicable catalytic platform for carbonyl-olefin metathesis. Achievement of this goal would enable the development of a range of valuable processes, including ring-opening and ring-closing carbonyl-olefin metathesis, carbonyl olefination, and non-metal based olefin metathesis. Funding from this ACS-PRF grant has been crucial to the viability of this research program.
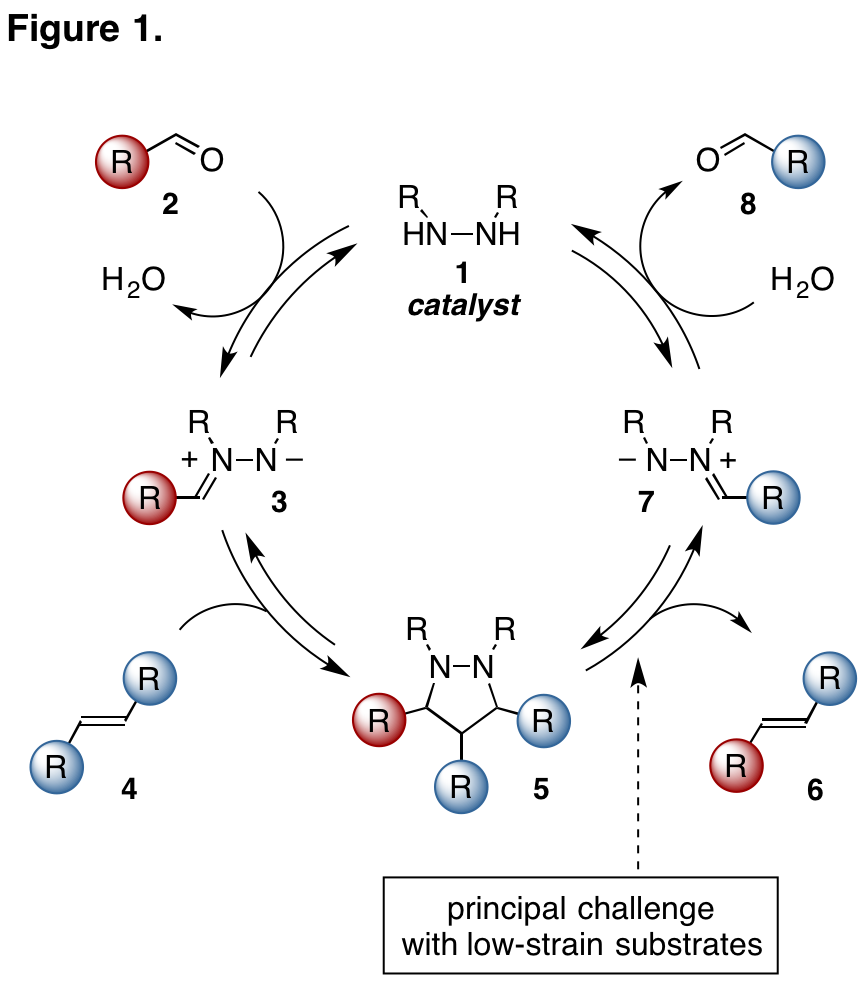
In brief, our group has developed a strategy for catalytic carbonyl-olefin metathesis using simple hydrazine catalysts. This strategy relies on a [3+2] cycloaddition / cycloreversion strategy, which thus represents a new paradigm for double bond metathesis (Figure 1). Our first implementation of this strategy involved the metathesis of aryl aldehydes and cyclopropenes. The cyclopropenes were necessary because their high ring strain provided substantial acceleration of both the cycloaddition and especially the cycloreversion steps. Indeed, for cyclopropenes, cycloaddition was found to be the rate-determining step, but for most other olefin substrates, cycloreversion was calculated to be rate-limiting, usually to a very large extent.
With funding from the PRF, we have identified several catalyst modifications that accelerate the cycloreversion step with less-strained olefin substrates (Figure 2). Among these, we have found that angle strain is an important structural variable, specifically that catalysts with larger C–N–N bond angles that will favor the all sp2-hybridized cycloreversion products (with preferred angles of ~120¼) while destabilizing the all sp3-hybridized cycloadducts (with preferred angles of ~109¼). We have also found that steric congestion also correlates to increase cycloreversion rates, suggesting that catalyst substitution may offer enhanced reactivity. Finally, we have identified electronically-modified catalysts that provide massive acceleration for the cycloreversion step that should lead to the development of a significant expansion of catalytic carbonyl-olefin metathesis chemistry. This latter effect represents the primary focus of our research moving forward.
We have also investigated a variety of step-wise double bond metathesis reactions that have significant implications for complex molecule synthesis. These reactions make use of simple and widely available reactants and thus should offer a general menu of new methods in this area.
This ACS-PRF grant has helped to support the training of five different graduate students working toward their Ph.D. degrees, three during the current period. Through their efforts on this program, these students received training in the techniques of organic synthesis, including reaction set up and workup, product purification, data collection and analysis, and the logic of project development. Two of these students have graduated with their Ph.D.s, with this PRF-funded research serving as a significant portion of their thesis work. Of the two other students still working toward their degrees, one was able to begin her graduate studies this past summer because of the funding from this grant, and her thesis work will continue to build on the foundation the PRF funding has provided.