Reports: DNI554706-DNI5: Role of Surfaces and Surface Charge on Hydrate Nucleation and Adhesion
Vaibhav Bahadur, PhD, The University of Texas At Austin
Summary:
Over the past year, grant funds have been used to build two experimental setups and conduct experimental studies. Two PhD students and two undergraduate students were supported. Two PhD students and 1 undergraduate student graduated during this year. Over the last year, this work resulted in 2 journal articles, and 2 articles in conference proceedings.
Introduction:
Formation and buildup of natural gas hydrates in deepwater infrastructure is a big challenge faced by the petroleum industry. Hydrates also have other applications in natural gas transport, carbon sequestration, and desalination. This work aims to uncover the role of surface chemistry and surface charge on hydrate nucleation and adhesion. Year 2 progress can be described via 3 studies.
Study 1 (completed): Electro-nucleation of tetrahydrofuran (THF) hydrates
Electro-nucleation is electrically-induced nucleation of crystals from a supercooled liquid. As a precursor to studying the role of surface charge on nucleation of methane hydrates, an experimental study was conducted to study electro-nucleation of tetrahydrofuran (THF) hydrates. The objective was to gain experience into measurements of nucleation and train a graduate student for more complex experiments with methane hydrates.
THF (C4H8O) forms structure II hydrates with water from a mixture of THF and water (stoichiometric molar ratio between THF and water is 1:17) at atmospheric pressure and at temperatures below 4.4°C. THF hydrates are commonly used as model hydrates for methane hydrates (which form under high pressure conditions of ~75 atmospheres), since they have a similar structure.
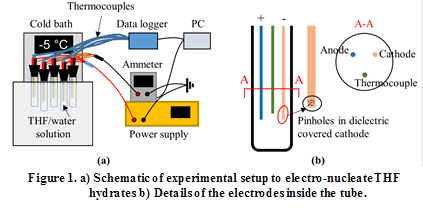
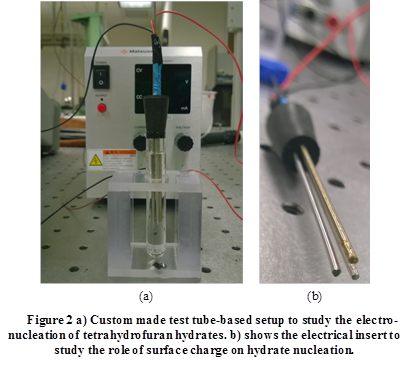
Figure 1 shows a schematic of the experimental setup and Figure 2 shows a picture of the setup. Hydrate formation was studied in glass tubes immersed in a cold bath. Stainless steel electrodes and a thermocouple were inserted into the tube, as shown in Figure 1(b). The electrodes were connected to a DC power supply and an ammeter. A custom holder was fabricated to hold and immerse multiple tubes in the bath. Within each tube, the cathode was dip coated with a dielectric layer, with pinholes intentionally created in the dielectric layer. The objective behind the dielectric layer with pinholes is to restrict and control the amount of current flow.
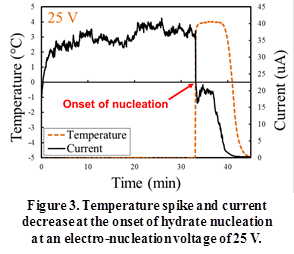
The onset of nucleation was detected as a temperature spike (Figure 3) by the thermocouple. The water-THF system releases heat (heat of formation of hydrate) to reach the equilibrium temperature for hydrate formation (~ 4 °C). The second indicator of hydrate nucleation is a sudden decrease in the electrical conductivity of the solution (Figure 3).
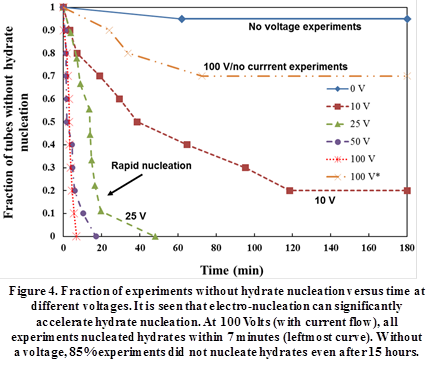
Figure 4 shows the fraction of tubes that did not nucleate hydrates versus time for various voltages. Results of the control experiments (no voltage) show that more than 95% tubes did not nucleate hydrates even after 180 minutes. In contrast, all the tubes at 100 V showed hydrate nucleation within 7 minutes. Increased voltages reduced the induction time, with all tubes nucleating in less than 50 and 18 minutes at 25 and 50 V, respectively. Figure 4 clearly highlights the utility of electro-nucleation and indicates that electro-nucleation can reduce the induction time by more than 100X.
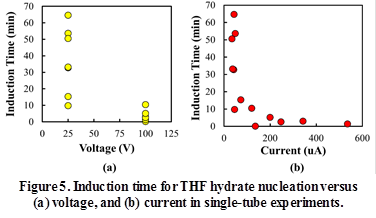
Figure 5 shows the induction time versus current and voltage for single tubes. The induction time decreases with higher voltages and currents. The induction times at 100 V are considerably lower than those at 25 V. Furthermore, the spread in measured induction times is also lower at 100 V.
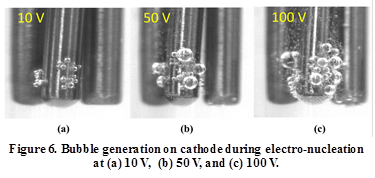
The mechanisms underlying electro-nucleation were briefly analyzed. Current flow in the solution leads to electrolysis at the pinhole sites; this will generate hydrogen bubbles, which act as nucleation sites to increase the probability of nucleation. This hypothesis was validated by separate visualization experiments to obtain qualitative comparisons of bubble formation at various voltages. Figure 6 shows the difference in the size and distribution of bubbles on the cathode at 10, 50, and 100 V. As expected, the amount of bubbles (number and size) on the cathode increased with voltage.
Study 2 (ongoing): Metal foams for rapid, low voltage electro-nucleation of tetrahydrofuran (THF) hydrates
The use of high porosity metal foams can lead to rapid electro-nucleation at very low voltages, resulting from the very high density of nucleation sites in the foams. Additionally, metal foams can rapidly dissipate the heat released during hydrate nucleation, and accelerate the hydrate growth phase.
An ongoing study is using metal foams as one of the electrodes to induce rapid electro-nucleation of THF hydrates at voltages less than 10 Volts. An image of the foam and the experimental setup is shown in Figure 7. Preliminary results indicate that voltages as low as 5-10 V can trigger electro-nucleation in a few minutes.
Study 3 (ongoing): Studies on the role of surface chemistry and surface charge on methane hydrate nucleation
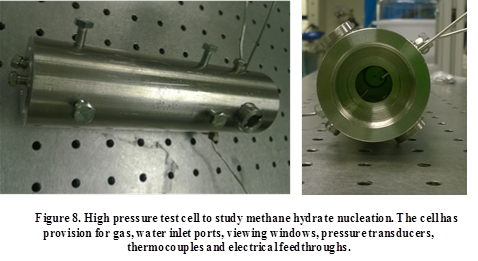
Figure 8 shows a custom-built experimental setup which is being developed to study the role of surface chemistry and surface charge on methane hydrate formation. This consists of a high pressure cell (rated to 125 bar) which can be filled with natural gas and water; the entire cell is immersed in a cold bath (-10 ° C).
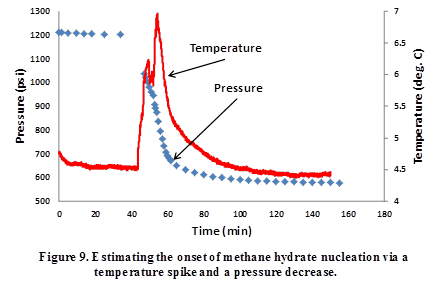
Experiments have been conducted to synthesize methane hydrates in the above cell. Figure 9 shows a case of successful methane hydrate nucleation. The onset of nucleation is detected by a temperature spike (heat of formation released) as well as a pressure drop (due to consumption of methane gas).
Ongoing studies are aimed at studying the influence of surface chemistry via the use of chemically functionalized metal foams inside the cell. The role of surface charge will be studied by applying an electric field across the foam electrodes.
Impact of project work
In the past year, 2 students working on this project graduated with PhDs in Mechanical and Chemical Engineering, and will now work for Raytheon (thermal management group) and Frito Lay (food science group) respectively. An undergraduate student, working on the experimental setup, graduated with a BS degree in Mechanical Engineering and is currently enrolled in the graduate program at USC.