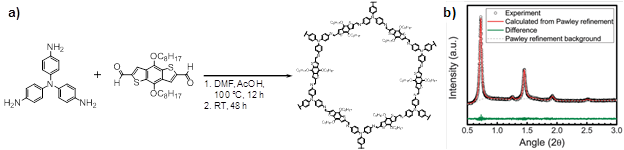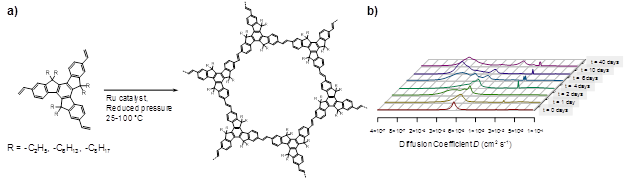Reports: ND1054335-ND10: Synthesis, Functionalization, and Processing of Conjugated Microporous Polymers for Refinery Stream Separation and Purification
Zhenan Bao, Stanford University
During the first reporting period of this grant (2014-2015), we identified and synthesized a conjugated microporous polymer (CMP) system for potential applications in refinery fluid separations. We discovered a novel method to form thin films of the material; thicknesses between ~2-200 nm were achieved. Structural characterization showed that this material, termed “polyTB,” contains a regular array of large (~2.9 nm) hexagonal pores in a honeycomb-like pattern (Figure 1). While this material was successfully applied to semiconductor devices, it lost porosity on drying, rendering it less effective as a membrane for separations. Hence, our activities in this reporting period (2015-2016) focused on seeking understanding film formation and applying this understanding to the design and synthesis of more robust, porous CMPs.
Figure 1. a) Synthetic scheme for semiconducting polyTB film (DMF = N,N-dimethylformamide). b) Synchrotron X-ray powder diffraction (λ = 0.414 Å) and Pawley refinement against a computational model of polyTB.
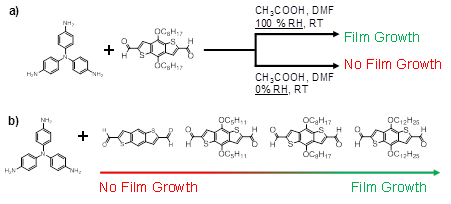
Investigations into polyTB analogues with varying side-chain lengths affixed to the aldehyde monomer (Figure 2) previously suggested that film formation could be influenced by the hydrophobicity of the monomers. Linear aliphatic side-chains having twelve or eight carbon atoms led to the formation of thin films; thicker films were obtained when using monomers having longer side-chains. In contrast, no films could be formed when synthesizing polyTB having either five or zero (no side chain) carbon atoms in monomer side-chains. Along with further experiments varying humidity during film growth, we hypothesized that water diffusion into the dimethylformamide-based growth medium partitioned hydrophobic monomers and growing oligomers to the interfaces of the growth vessel, including the air-solution interface. Thus, polymer films easily processed by physical transfer methods could be obtained.
Figure 2. Schematic showing conditions under which thin (~2-200 nm) films of polyTB and analogues can be grown at the solution-air interface.
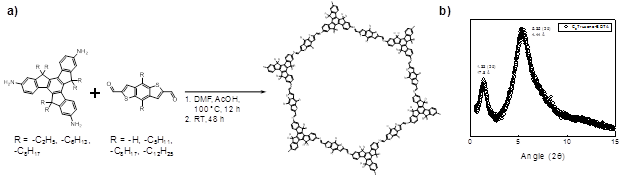
Both to obtain more robust polymers and to further understand film growth, we selected truxene-based monomers (Figure 3a) to synthesize the next set of CMPs. Exploration of olefin metathesis as a means to synthesize truxene-based CMPs by homopolymerization was successful in forming mixtures of network oligomers (likely polydisperse, ring-containing molecules), as evidenced by gel-permeation chromatography, light-scattering, and diffusion-ordered NMR experiments (Figure 3b). However, despite efforts to increase the extent of polymerization, no materials could be obtained from the reaction of these monomers. Hence, we returned to the original approach, whereby imine condensation could potentially yield CMPs. By functionalizing truxene monomers having side-chains with two, six, or eight carbon atoms (Figure 4), we synthesized a series of truxene-based CMPs and studied their film-forming properties. We found that combination of the all truxenes with the eight-carbon side-chain containing aldehyde formed thin, processable films. Furthermore, reaction of side-chain-free aldehyde monomers still yielded films when using truxenes having side chain lengths of six or eight carbon atoms. In contrast, truxene monomers having side-chains of two carbon atoms were unable to form films with the side-chain-free aldehyde monomers. These results show that our novel film-forming method is applicable to other CMP materials, and may potentially be general to many more condensation-based network polymers. They also underscore the importance of selecting appropriately hydrophobic side-chains for film formation and are consistent with the mechanism for film formation we developed for polyTB and related materials. Despite the selection of truxene as a polymeric subunit less prone to oxidation and ring twisting than the tris(4-amino phenyl)amine used in polyTB, truxene films still exhibited a loss in order on drying.
Figure 3. a) Scheme for synthesis of truxene-based network polymers (structure of product is an idealized model). Ru catalysts tested include Grubbs 2nd and 3rd generation catalysts, as well as the 1st generation Hoveyda-Grubbs catalyst. b) Diffusion-ordered (DOSY) 1H NMR showing distribution of reaction products over time. Smaller diffusion coefficients correspond to higher molecular weight products.
Figure 4. a) Scheme for synthesis of truxene-based polymer films by imine condensation. b) Synchrotron powder X-ray diffraction of dry truxene-based polymer (λ = 0.414 Å). Broad peaks indicate significant disorder compared with polyTB and analogues (see Figure 1b).
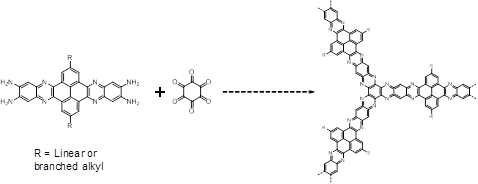
As conjugation may lead to higher thermal conductivities than those possible in typically insulating high surface area materials, we are continuing to explore routes to porous, conjugated, thin-film forming materials. We are currently designing monomers that may yield robust, fused-ring network polymers via dione-diamine conjugation (Figure 5) as discussed in the initial proposal. Monomers are being designed that will be long enough to lead to nm-diameter hexagonal pores, but having the hydrophobic side-chains necessary both for monomer solubility and for the formation of processable thin films via ambient water diffusion into the film growth medium.
Overall, the results from this work have established a new film-forming method applicable to CMPs in general, and potentially other condensation-based polymers. These results have already proven successful for forming high-quality thin films suitable for organic electronic device applications; while guest-accessible porosity has been difficult to achieve in dried films, the use of more robust, fused-ring monomers and network formation chemistry is expected to lead to membranes having the uniform porosity and robustness needed for molecular separations.
Figure 5. Proposed synthesis for robust, fused-ring CMPs.
Education and Outreach: The results from the postdoctoral scholar heading the project were delivered in invited seminars at the University of Delaware and the National Institute of Standards and Technology, publicizing the work to both academic and government researchers. Furthermore, the results were discussed in a Gordon Conference (“Crystal Engineering,” Stoweflake Conference Center, Stowe, VT, June 26 – July 1, 2016) attended by the postdoctoral scholar.