Reports: UR354579-UR3: BODIPY-Pd Complexes for Photocatalytic C-C Coupling Reactions
Hongshan He, PhD, Eastern Illinois University
The objective of this project is to test the hypotheses that BODIPY (BODIPY= 4,4'-difluoro-4-bora-3a,4a-diaza-s-indacene) chromophore can activate Pd(II) through an intramolecular electron transfer process for catalytic Sonogashira C-C coupling reaction under visible light illumination. We plan to prepare new BODIPY-modified 1,10-phenanthroline ligands and their Pd(II) complexes to test this hypothesis. The absorption, fluorescence, and electrochemical properties will be determined to reveal the energy transfer process. The theoretic calculations will be performed to provide insights on the energetics of intermediates. Detailed photocatalytic properties of the Sonogashira C-C coupling reactions will be investigated to reveal the reaction mechanism.
In Year 2, the graduate continues to work on the project and one undergraduate student participated in the project during the summer of 2016. Both students obtained significant training on manipulation of air-sensitive materials sing Schlenk lines and glovebox. Our major focus during this period is to study the catalytic reactions under different conditions. Some of these results have been presented at the ACS 2016 Fall Meeting in Philadelphia. A manuscript is currently under preparation for publication.
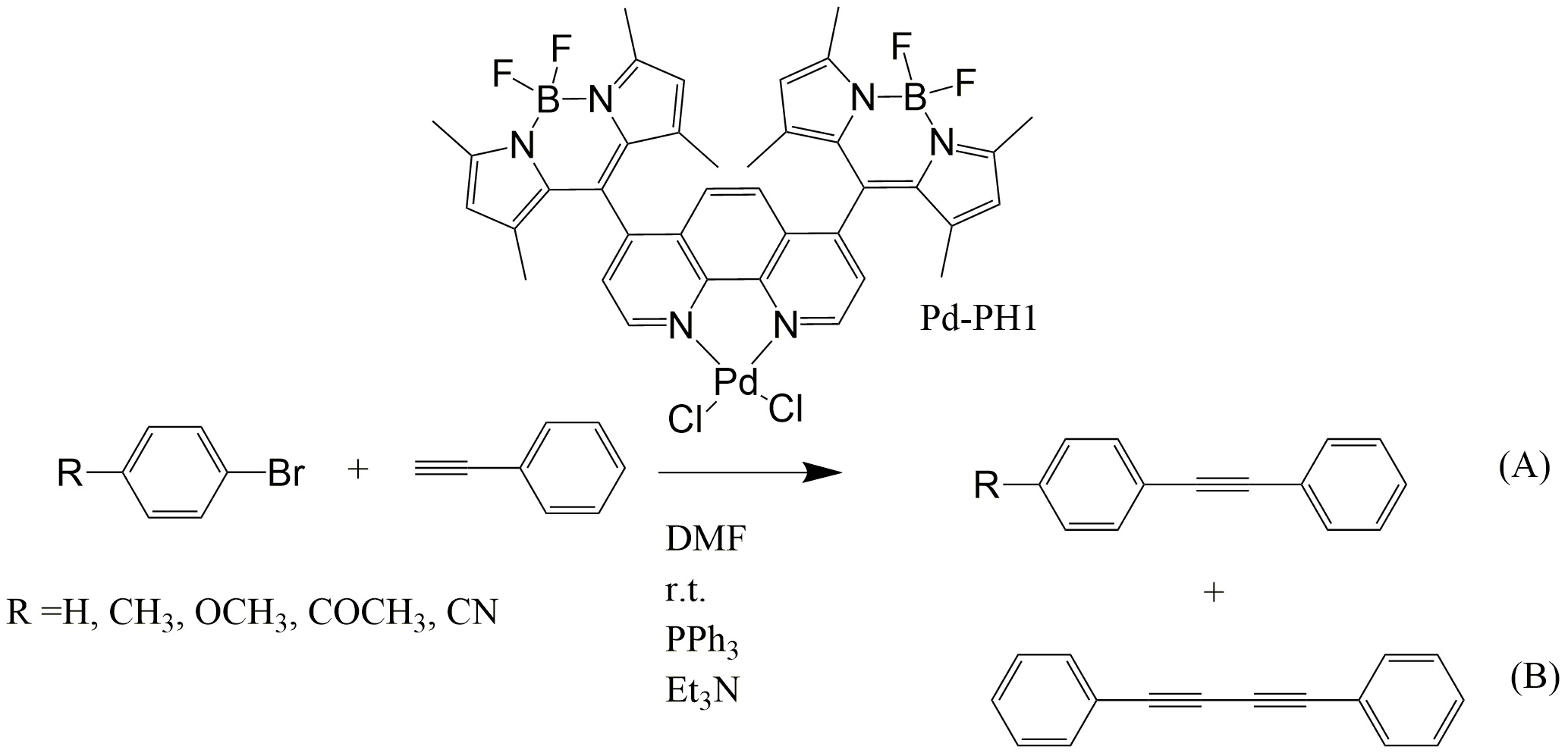
1. Catalytic studies. As planned, we carried out a series of catalytic reactions as depicted in Figure 1. We selected four different bromides with R groups exhibiting either electron donating or withdrawing properties. First we run a reaction under conventional Sonogashira condition. We found that Pd-PH1 can catalyze the reaction in the dark when CuI was used as a co-catalyst under anaerobic conditions. In addition to the expected cross-coupling product A, a homo-coupling product (B) was also observed. Both are confirmed by GS-MS. In the absence of CuI, only homo-coupling product (B) was observed under dark (without light irradiation) and under light illumination (anaerobic or aerobic). No cross-coupling product was observed. This indicated that Pd-PH1 functioned as an efficient homo-coupling reaction catalyst. We In the year 3, we plan to study the reaction mechanism of the catalytic reaction and try to vary the reaction conditions to increase the cross-coupling yield.
Figure 1. Pd-Ph1 catalyzed reactions
2. Fluorescence of ligands and Pd (II) complexes. We also studied the fluorescence spectroscopic properties of ligand and complex using our newly purchased fluorescence spectrometer FS5. Previously we found that the ligand showed very strong fluorescence under UV illumination; the fluorescence was almost completely quenched in the Pd (II) complex as. This indicated clearly that Pd (II) induced or altered the fluorescence decay pathways. It is unclear at this moment if this is caused by electron transfer from BODIPY to Pd (II) center. We studied the fluorescence using our new instrument and found the decay lifetime for PH1 is 8 ns, where as in Pd-PH1, it was less than 1 ns. This is indicative of the electron transfer process from BODIPY core to Pd ion. We plan to perform some CV measurements to understand the oxidation and reduction process of Pd under light irradiation.
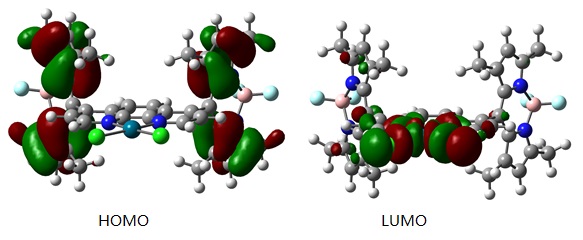
3. Theoretical calculations for two ligands were carried out at XSEDE facilities. We submitted an XSEDE and was awarded a grant to perform high-performance computing on Comet at SDSC through the grant TG-CHE130116. The calculations were performed on density functional theory level using B3LYP/6-31g(d) in acetonitrile.4 The optimized structures were very similar to the single-crystal structures. The electron density of HOMO is centered on BODIPY whereas the LUMO is on the Pd center indicating that BODIPY unit will be most likely the electron provider for the reduction of Pd (II).
Figure 2. Frontier molecular orbital of Pd-PH1.