Reports: ND555270-ND5: Low Temperature Activation of Methane
Feng Jiao, University of Delaware
Narrative Report
Methane is a plentiful, naturally occurring hydrocarbon, and the main constituent of natural gas. Due to its abundance, it has been well studied as both a feedstock for chemical production and as a fuel. Methane has also become the subject of research as of late because it is often released into the atmosphere as a result of human activities such as coal mining, natural gas petroleum drilling, and breakdown of garbage of landfills. These human activities account for over 60% of all methane emissions into the atmosphere. The highest percentage of emissions come from oil and natural gas sites, which account for 34% of all US methane emissions. The key challenge related to methane conversion to other high-value chemicals is the high C-H bond energy, which is >100 kCal mol-1, the highest bond energy between carbon and hydrogen among hydrocarbons. This high bond energy gives methane great stability and thus difficulties in partial oxidation.
Current State-of-art low-temperature methane activation processes involve the use of hydrogen peroxide as an oxidant. The process showed a good selectivity towards methanol under hydrothermal conditions. In the first year of this project, we have studied the electrochemical hydrogen peroxide generation process that can be coupled with the partial methanol oxidation reaction to produce methanol. In the initial investigation, we studied nitrogen doped carbon (NDC) as potential oxygen reduction catalyst for selective hydrogen peroxide production. NDC catalysts are of high interest because they are metal free, easily synthesized, and exhibit good selectivity in oxygen reduction. In the past 12 months, we have successfully synthesized mesoporous NDC catalysts using the nanocasting technique. In a typical synthesis of mesoporous NDC catalyst 2g of silica (KIT-6) was mixed with 5 g of de-ionic water and 2 g of an ionic liquid, 1-Ethyl-3-methylimidazolium ethyl sulfate (EMIM-SO4, >95% Sigma-Aldrich). The ionic liquid served as the carbon and nitrogen sources for the NDC catalyst. The mixture was transferred to an alumina crucible and calcined for 1 hour at 1073 K under N2 flow. The heating rate was 10 K min-1 and the system was purged with N2 for 1 hour before heating. The crucible and mixture was allowed to cool overnight in the tube. The hard silica template was removed by washing in a 3M NaOH solution.
To prepare the electrode, 15 µL of NDC was dropcasted onto a 5mm OD glassy carbon (GC) electrode (Pine) from the following solution: 10mg NDC, 1.99 mL DI water, 0.5 mL 2-Propanol (Certified ACS, Fisher Scientific), 10 µL Nafion solution (Sigma-Aldrich). The solution was dried on a pre-polished GC electrode for 15min at 333 K. Polishing was done with 0.05 µm alumina solution on an Eco-Met 250 (Buehler). In order to evaluate the catalytic performance, a Princeton Applied Research VMP 2 potentiostat was used with a 3-electrode RDE setup. RPM was controlled by a MSRX control box (Pine). The counter electrode was a graphite rod. The electrolyte was 0.5 M H2SO4 (Certified ACS+, Fisher Scientific) or 0.1M HClO4 (ACS, Sigma-Aldrich). Current was measured against a Ag/AgCl reference electrode (Pine) and converted to the Reversible Hydrogen Electrode Scale (RHE).
CV scans were carried out in oxygen and argon rich electrolyte and the resulting current was subtracted to eliminate background effects. The mesoporous NDC catalyst has an onset potential of 0.55V (RHE) compared to 0.45 V (RHE) for the two catalysts from literature. At a potential of -0.1 V (RHE) our catalyst had a limiting current density of ~3.7 mA cm-2. The results clearly show that the performance of mesoporous NDC is comparable to the state-of-art catalysts that were recently reported in the literature.
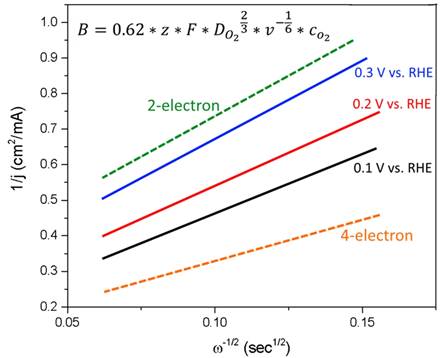
Koutecky-Levich plots were generated to determine selectivity of the NDC towards H2O2. For a 4-electron process B =0.43 mA cm-2 s0.5 while for a 2-electron process B=0.21 mA cm-2 s0.5. The plot shows that at 0.3 V, the slope for mesoporous NDC is in good agreement with the theoretical slope for 2-electron transfer. This indicates that our catalyst selectively produces H2O2 at that potential. As the overpotential increases the slope of the lines gradually shifts away from 2-electron transfer to 4-electron transfer.
In the second year of this project, we will couple the electrochemical hydrogen peroxide production process with the partially oxidize methane reaction. Previous literature has shown that Fe-ZSM-5 is an active catalyst for methane activation using hydrogen peroxide as the oxidant. We will investigate the possibility to use iron-based catalyst in an integrated electrochemical cell to produce valuable chemicals, such as methanol, in a single step process.