Reports: DNI153703-DNI1: Cobalt-Catalyzed Carbon-Carbon Bond Forming Reactions of Simple Olefins
Thomas J. Maimone, PhD, University of California, Berkeley
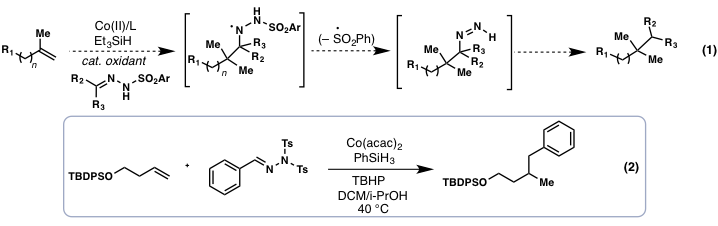
The unifying theme of our research has been the study of metal-catalyzed radical reactions. Such reactions hold enormous potential for the conversion of simple chemicals, including feedstock olefins, into more complex, higher value products. Metal-catalyzed olefin hydrofunctionalization, as originally developed by Mukaiyama and Isayama using Cobalt catalysis, has recently witnessed an explosion in growth for the construction of C–X bonds (X = F, Cl, O, H, N, S) from simple alkenes (see: Chem Rev. 2016, 116, 8912). At the start of our work, however, only a single report detailed useful C–C bond forming processes using this radical-based manifold, Carreira's hydooximation reaction. Very recently, several new C-C bond-forming processes have begun to emerge. We proposed, the development of two novel, metal-catalyzed hydroalkylation and hydroarylation reactions. In the first transformation, we sought to develop simple hydrazones as reagents that can transfer alkyl groups to alkenes in accordance with equation 1.
During the grant period we prepared and evaluated numerous aldehyde and ketone-derived hydrazones in an effort to indentify suitable alkyl transfer reagents and conditions for this process. While out results obtained indicated this challenging transformation is possible in a single step (equation 2), we were unable to optimize this transformation above ~5% yield (determined by gas chromatography). Notably, during the course of our studies, a report by Baran and co-workers described a highly similar transformation using Fe-catalysis, yet this was limited to only formaldehyde-based hydrazones. It appears the steric constraints surrounding larger hydrazones precludes efficient coupling. These independent studies also highlighted the difficulty of a one-pot nitrogen extrusion step, something that also likely contributed to inefficiencies in our process.
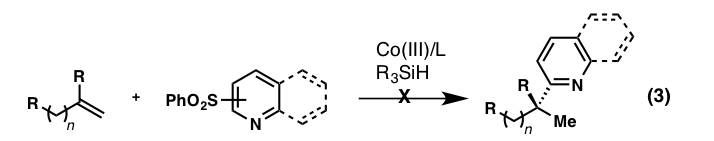
A second process that was originally proposed and explored was the direct coupling of heteroaryl sulfones with unactivated alkenes, which was envisioned to be an extension of the previously mentioned hydrooximation reaction developed by Carreira and a valuable synthetic alternative to the Friedel-Crafts reaction (equation 3). We were unable to realize success in this direct, intermolecular coupling reaction, in line with recent studies by Shenvi.
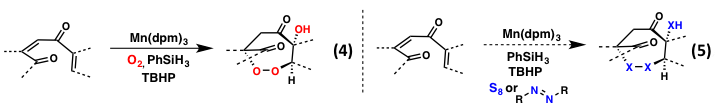
These studies, and the in-house knowledge they have brought to my group, have been instrumental in continuing with our laboratories interest in alkene functionalization chemistry using inexpensive transition metals (Co, Mn, Fe). We have has also recently discovered a novel peroxidation cascade under Mn-catalysis leading to complex, 7-membered endoperoxide scaffolds from simple starting materials (equation 4); such motifs have been challenging to form in comparison with their 5- and 6-membered counterparts. We have also attempted to extend this chemistry toward polyamination and thiolation (equation 4), but have been unsuccessful to date using using previously developed Boc hydrazine derivatives and elemental sulfur.
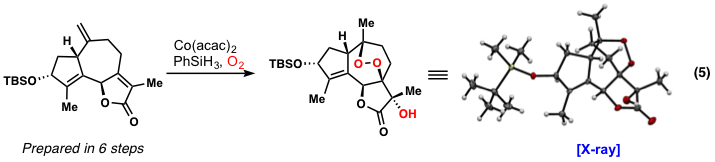
These results have lead us to further explore polyoxygenation cascasdes in more complex and challenging frameworks, such as those found in the guaianolide sesquiterpenes. In a model study, much of the oxygenation present in this family of compounds could be installed in a single step under Co-catalysis (equation 5). We are currently optimizing this process for use in terpene synthesis as the present yields are still low (~10-15%).
We are highly appreciative to the ACS PRF for their support of our work, which has had a profound effect on the PI's early career. This support has allowed the PI to substantially broaden his research portfolio. While the originally intended transformations have yet to translate into mature and usable synthetic methods, much of the PI's research involving radical-based transformations have been influenced by the early ACS PRF funding and many of the key concepts and ideas were seeded during these initial explorations. The funding by the ACS PRF has also led to the scientific development and training (both intellectual and experimental) of future synthetic chemists.