Reports: DNI1054544-DNI10: Investigating Polymer Templated Graphene Scaffold for Heterogeneous Gold Nanoparticle Catalyst in Selective Oxidation Reactions
Yu Zhu, PhD, University of Akron
Introduction
The central thesis of this research is to synthesize and characterize novel heterogeneous catalysts with ultrasmall gold nanoparticles (NPs) on organized graphene scaffolds for selective oxidations. In this research, two-dimensional materials are used as multifunctional substrate, which not only provide scaffolds for small gold NPs, but also guide the formation of nanoparticles to obtain well-organized, uniform nanoparticle arrays. Such a novel hybrid system may provide new opportunities to develop selective oxidation reaction catalysts.
The major goals during this reporting period were (1) to integrate gold NPs with 2D materials (graphene and molybdenum disulfide) and (2) to study the catalytic effect of gold NPs/2D materials in selective oxidation reactions.
Accomplishments
Integration of gold NPs and graphene
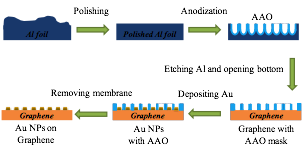
In the previous report period, we demonstrated a method to fabricate free standing AAO (anodic aluminum oxide) membrane. This method was further developed in the past year to deposit small gold NP array on the 2D material substrate. The fabrication process is shown in Scheme 1.
Scheme 1 Schematic illustration of fabricating gold NP array on graphene.
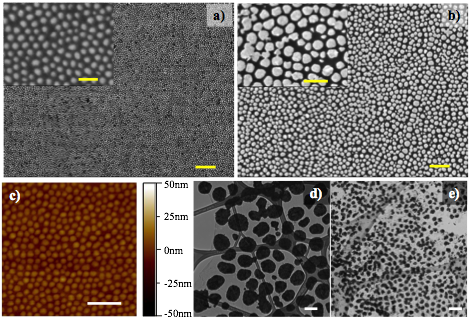
The morphology of gold NPs on graphene was characterized by SEM and AFM. As shown in Fig. 1a-c, the gold NPs are uniformly distributed on the entire surface. The freestanding gold NPs on graphene samples were prepared as well, which allowed TEM characterization. The TEM images of large gold NPs (Fig. 1d) and small gold NPs (Fig. 1e) on graphene clearly confirmed that such a well-organized gold NPs modified graphene can be prepared by facile deposition method. As far as we know, this is the first report of organized gold NPs modified free-standing graphene. [1]
Fig. 1 a, b) SEM images of gold NPs on graphene (the insets are same samples under higher magnification), the scale bar in is 1 µm. The scale bars of the insets are 200 nm (a) and 500 nm (b), respectively. c) AFM image of gold NPs on graphene surface (scale bar: 500 nm) d, e) TEM images of gold NPs with different size on free-standing graphene (scale bar: 200 nm)
A subsidiary finding of this research is that the gold NPs modified graphene can serve as a conductive, SERS (Surface Enhanced Raman Spectroscopy)-active substrate. This resulted in a side project of utilizing the SERS-active electrode to analyze electrochemical reactions. The electrochemical selective oxidation process, such as the ORR (oxygen reduction reaction) process, was investigated. [1] As shown in Fig. 2, the discharging products on SERS-active electrode are Li2O2 and Li2CO3, however, the Raman spectroscopy of conventional electrode can not provide any chemical information.
Fig. 2 Fabrication and testing of lithium-air cell with SERS cathode. a) Illustration of the device: SERS cathode (WE), Li foil (RE and CE) b) The picture of the device c) Raman spectra of different cathodes after discharging
Integration of gold NPs and MoS2
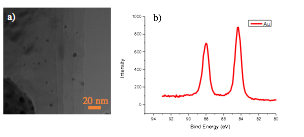
In the past year, we also investigated another 2D material, molybdenum disulfide, as scaffold material for heterogeneous catalysts. Molybdenum disulfide is a semiconductor with bandgap over 1.2 eV. The direct contact of gold atom with MoS2 will change the electronic state of gold, therefore the catalytic activity may be enhanced. [2] In the first annual report, we reported the synthesis of gold NPs from HAuCl4 solution. Similar technique was used to synthesize gold NPs on MoS2. As shown in Fig. 3, the gold nano particles modified MoS2 was successfully synthesized. The XPS results indicated the formation of metallic gold (Au 4f7/2 at 84.3 eV) on MoS2.
Figure 3. a) The TEM image of gold NPs on MoS2. b) The XPS (Au 4f7/2) of gold NPs modified MoS2.
To investigate the catalytic effects of gold NPs/2D substrate in selective oxidation reactions
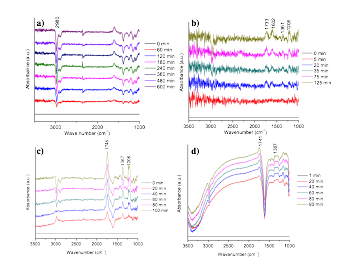
Selective oxidation of isopropanol was used to evaluate the catalysts. To monitor the reaction process, an in-situ IR reactor was used. Oxygen gas was purged through an isopropanol bath into the reaction chamber. The purging lasted for a few minutes to ensure the coverage of isopropanol molecules on catalyst surface. The cell was then sealed and IR spectra were recorded over time. From Fig. 4a, the peak at 2980 cm-1 is assigned to C-H bond, which decreases during the reaction, suggesting the consumption of isopropanol. Meanwhile, the peaks at 1733, 1361 and 1206 cm-1 increase, indicating the formation of acetone. The peak at 1606 cm-1 can be assigned to the dehydration of acetone to form mesityl oxide. [3] The existence of UV source was found to accelerate the oxidation reaction (Fig. 4c). The control experiments were conducted by using bare MoS2 without Au NPs. The results (Fig. 4d) indicated the reaction rate is very slow.
Figure 4 a, b) The subtracted In-situ FTIR spectra of isopropanol selective oxidation over time (using 0 min spectrum as baseline) c) In-situ FTIR spectra of isopropanol selective oxidation over time with UV source. d) in-situ FTIR spectra of control experiment (MoS2 without Au NPs).
Educational efforts from this research
Graduate students funded by the ACS PRF grant fabricated Au NPs/graphene and Au NPs/MoS2 catalysts. One post-doc was partially supported by this project to conduct the in-situ IR study. They worked together with two self-supported MS students and one ACS SEED student to synthesize small gold NPs. Through this study, they received a broad training experience, acquired fundamental knowledge of nanoparticle synthesis and a suit of characterization tools. All students and post-docs assisted in manuscript preparation and one graduate student presented the work at Carbon Conferences 2016.
Future Plan
In the future, we will continue to explore the catalytic performance of Au/MoS2 system in selective oxidation reactions. The study will focus on the understanding of reaction mechanism and characterization of the intermediate products.
[1] K.W. Liu, Z.T. Yu, X.W. Zhu, S. Zhang, F. Zou, Y. Zhu, RSC Adv. submitted
[2] A. Abad, P. Concepci—n, A. Corma, H. Garc’a, Angew. Chem. Int. Ed., 2005, 44, 4066–4069
[3] M. El-Maazawi, A.N. Finken, A.B. Nair, V.H. Grassian, Journal of Catalysis, 2000, 191, 138-146