Reports: DNI654371-DNI6: High Pressure Studies of Aromatic and Aliphatic Soot Formation Behind Reflected Shock Waves
Patrick Lynch, PhD, University of Michigan-Dearborn
Background and Experimental Approach:
The project involves studying the formation of soot from different precursors in high pressure conditions behind reflected shock waves, and trying to observe a synergistic effect on the reduction of soot from the mixture of different radical-pool-changing precursors. Gases and particles are continuously sampled by expansion into an endwall mounted vacuum chamber, and laser scattering is used to measure particle sizes and number density in a time resolved fashion, and also to form an indication of induction delay. The shock tube is a 12.7 mm bore high repetition rate shock tube (HRRST), which can be used to make repeatable measurements in conditions like this, a low signal-to-noise level experimental system which requires significant signal averaging.
Technical Progress:
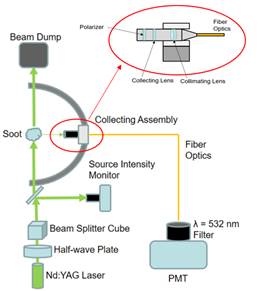
Since the previous technical report we were forced to make changes to the vacuum chamber goniometer in order to increase the sensitivity to small levels of scattered light from particles sampled behind reflected shocks into the vacuum chamber. This aspect of the work imposed a significant delay on scattering experiments, but an acceptable solution was found using fiber optics coupled to a high gain PMT (instead of an amplified photodiode). Significant effort went into redesigning the optical collection system mounted on the goniometer, as well as some custom vacuum chamber fiber feedthroughs. The improvements were substantial and permitted the PMT to measure the scattered intensity from these small particles.
Figure 1. Redesigned optics configuration in the goniometer.
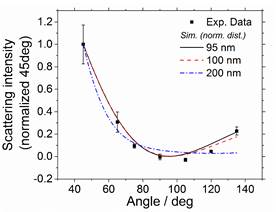
Figure 2 Normalized Scattering Transmitted Ratio at Different Angular Locations
After testing the new system by measuring the particle size of droplets in steam clouds, Fig. 2, we moved on to experiments in pyrolysis of fuels. The first experiments tested the pyrolysis of acetone behind reflected shocks. During experiments, the laser pulse is the master (heartbeat), but there was extensive jitter in the medical grade laser we were using as our light source, and large scatter in the time between the shock time of arrival at the endwall and the time the laser flashes on the particles (and the PMT measures the scattering). This is the “kinetic” time scale. This varies laser shot-to-shot partly from the laser and partly from variations in the shock velocity. Our solution was to embrace this large amount of scatter and use it for our time resolution. We simply track this kinetic time and bin by kinetic time for conditions with the same temperature and pressure history, and scattered angle. In this way, the time resolved data can be extracted from the experiments by binning in post-processing rather than just setting the kinetic time. This required a rewrite of the shock tube data acquisition software, and slight delay, but the resulting capabilities are extensive.
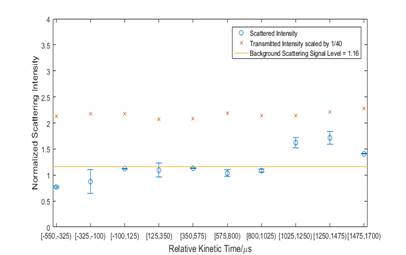
Figure 3. Normalized Scattering Intensity at Different Kinetic Time, T5 = 1560K, P5 = 10bar, 2%Acetone/Ar, Scattering Angle = 115°
Figure 3 shows an example of the scattered light intensity for one goniometer angle, temperature, and pressure, binned by kinetic time. The profile shows a lag between when the shock arrives at the endwall and when particles are observed in scattering. This lag is almost certainly because of the time lag in sampling gases from the endwall nozzle. The times labeled 1025 µs seem to be representative of particles formed at t=0. We have not tested enough conditions to apply this offset, but will do so.
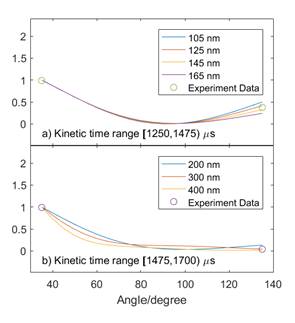
Figure 4 Mie Scattering Curve Fit at T5 = 1560K, P5 = 10bar, 2%Acetone/Ar. a) Kinetic time range between 1250 μs and 1475 μs. b) Kinetic time range between 1475 μs and 1700 μs.
Using time and condition resolved data obtained at different scattering angles, we can resolve particle size at different times. Figure 4 shows an example of this, and these sizes seem reasonable. The pyrolysis measurements will continue into FA16 (pyrolysis of ethylene and toluene) as well as the oxidative tests and tests with radical additives.
Related Progress and results:
During the delay as the optical system was redesigned to incorporate the PMT, we performed a chemical thermometry study to measure the temperature behind the reflected shock using tunable diode laser absorption spectroscopy. This was a useful step in validating the temperatures in subsequent studies. The rate of dissociation of 1,1,1-C2H3F3, was measured over a temperature range of 1250-1600K by monitoring the appearance rate of HF. This reaction rate was compared with literature rates. 1,1,1-C2H3F3 dissociates by HF elimination. The coproduct, 1,1-C2H2F2, is stable, except for a slow molecular elimination which also eliminates HF (again with well-studied rate coefficients). The HF R(1) transition was monitored with tunable diode laser (TDL) absorption. The chemical thermometry temperature was found by fitting the observable (concentration of HF) with simulation results using a detailed chemical kinetic model. Below 1450 K, there was agreement within experimental uncertainty between the temperature of the gases measured in this fashion and those calculated using the incident shock velocity and initial conditions. However the chemical thermometry temperature was lower (~40K) for higher temperature experiments (i.e. >1450K), indicating that there is some contribution from the sidewall boundary layers in those conditions.
Impact of Support:
Besides the technical impact, since the previous technical report, the program has provided partial support to two graduate students working on the project. One student has graduated with a MS, and another has defended his MS thesis but not yet deposited. Those students have both presented work in the past. In the past year, one student has presented at the Spring Technical Meeting of the Central States Section of the Combustion Institute work on the vacuum chamber goniometer and testing of the optical system. The PI has also presented work related to the chemical thermometry at both that meeting and the International Symposium on Combustion. One article has been published from work supported by this program, another in review, and two additional in preparation. The PI and students have already benefited from the extended capabilities to the laboratory after adding the new experiment. In addition to the intended scattering experiments, the endwall mounted chamber is used for other experiments.