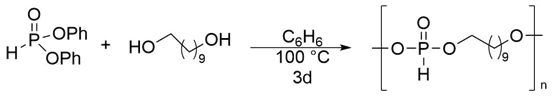Reports: UR151819-UR1: Phosphorus-Hydrogen Activation Using Alkynylmetal Complexes: New Methodology for the Preparation of Metallopolymers
Robert A. Stockland, Bucknell University
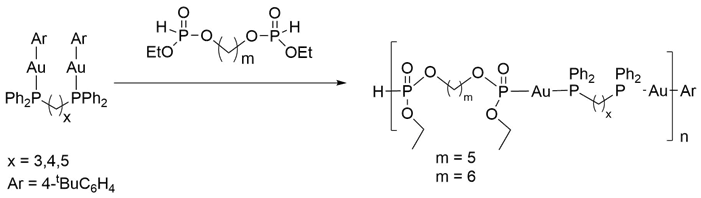
Scheme 1. Initial synthesis of metallopolymers.
Given the low solubility of the metallopolymers generated through the initial plan, we decided to pursue an alternate approach. While the initial methodology used protodeauration to link the monomers together, the new chemistry focused on a post-polymerization modification of a known polymer to incorporate the metal fragment. The prerequisite for this approach is the presence of reactive groups in the polymer or side chains that would facilitate binding of the metal center. While a number of functional groups could have accomplished this goal, we wanted to stay with the highly efficient protodeauration theme from our initial approach. To accomplish this, we needed a polymer containing secondary phosphites either in the pendant groups or backbone. During the initial stages of the project, we found that treatment of a diol with a large excess of a secondary phosphite generated mostly a bisphosphite compound that could be used as a linker (Scheme 1). If the amount of the phosphite is reduced to stoichiometric levels, oligomers and polymers are generated instead of monomers. Furthermore, while our intial studies used diethylphosphite as the source of the phosphorus component, we found that this reagent generated mostly oligomers and a broad polydispersity. Changing to diphenylphosphite solved this issue and the transesterification generated mostly polymers with DP = 40-50.
Scheme 2. Preparation of the polyphosphite.
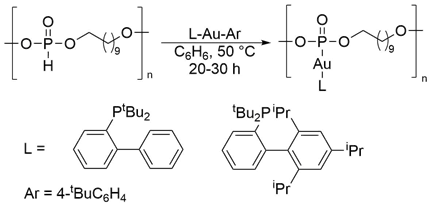
Treatment of the polyphosphites with LAuR complexes generated a metallopolymer where the metal fragment is essentially functioning as a pendent group. One of the advantages to this approach is the large number of highly functionalized monodentate phosphine ligands that are readily available. For example, Buchwald-type ligands are air-stable and can be tuned to increase the solubility of the resulting metal complex. To this end, we have used two such ligands to help increase the solubility of the metallopolymers (Scheme 3). Similar to our initial approach, the extent of metal incorporation could be determined by the amount of the free arene generated in the reaction, and high consumption of the arylgold compound was observed.
Scheme 3. Generation of the metallopolymers.
Impact on students involved in the project: During this phase of the project, I was very fortunate to have a number of very talented students working on the project. This project exposed them to a wide range of chemistry including the manipulation of air-sensitive compounds and reactions, careful use of NMR to determine the extent of reactions as well as the calculation of molecular weights based upon end group analysis. This project also gave them valuable experience with how to handle adversity when reactions do not proceed as intended. Further character building occurred when the products of all their efforts ended up being so insoluble that they could not characterize it. My students have also grown tremendously as scholars. They spent a significant amount of time trying to find reactions that would generate soluble precursors that would work for our chemistry. While most of my group are continuing students, one of the graduating seniors is pursuing further studies in chemistry in graduate school, while another is attending medical school.