Reports: ND555575-ND5: Supported Single-Atom and Subnanometer Pt Clusters for the Selective Oxidative Dehydrogenation of Ethane to Ethylene
Ayman M. Karim, PhD, Virginia Polytechnic Institute and State University
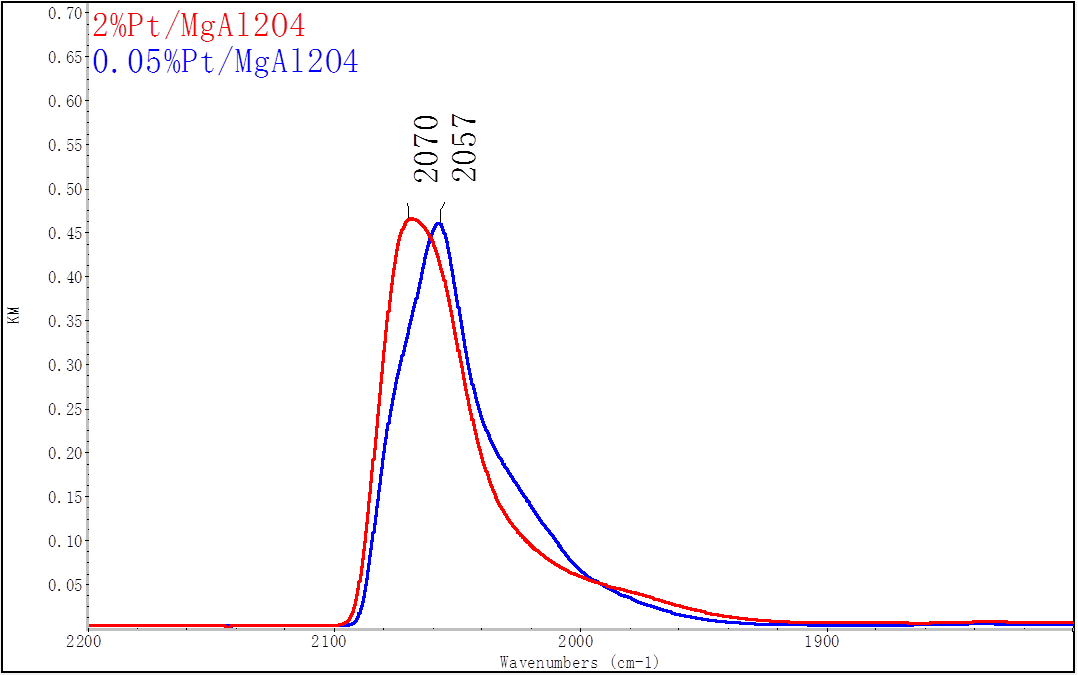
The goal of the project is to tune the selectivity of Pt for oxidative dehydrogenation of ethane to ethylene by controlling the metal nuclearity in the subnanometer regime. This is achieved by controlling the synthesis of Pt/MgAl2O4 using strong electrostatic adsorption (varying the pH, metal loading as well as the pretreatment conditions) to achieve Pt ensemble size ranging from single atoms to subnanometer clusters to nanoparticles. In the first year the work was focused on determining the synthesis and pretreatment conditions to achieve highly dispersed Pt in the form of single atoms and subnanometer clusters. Figure 1 shows DRIFTS spectra for CO adsorption on catalysts with different weight loadings. The peak at ~2070 and ~2057 cm-1 represent CO adsorbed on terrace and step sites, respectively. The spectra show that smaller clusters (lower Pt loading) show a smaller peak for CO adsorbed on terrace sites (2070 cm-1). Additionally, the smaller nanoparticles showed less reconstruction, i.e. change in the terrace/step peak ratio, at high CO partial pressure (not shown).
Figure 1: DRIFTS spectra for CO adsorption on 2% and 0.05%Pt/MgAl2O4 catalysts prepared by incipient wetness impregnation both calcined at 500 °C and reduced at 400 °C. The peak at 2070 and ~2057 cm-1 represent CO adsorbed on terrace and step sites, respectively. The spectra show that smaller clusters have a smaller peak for CO adsorbed on terrace sites (2070 cm-1).
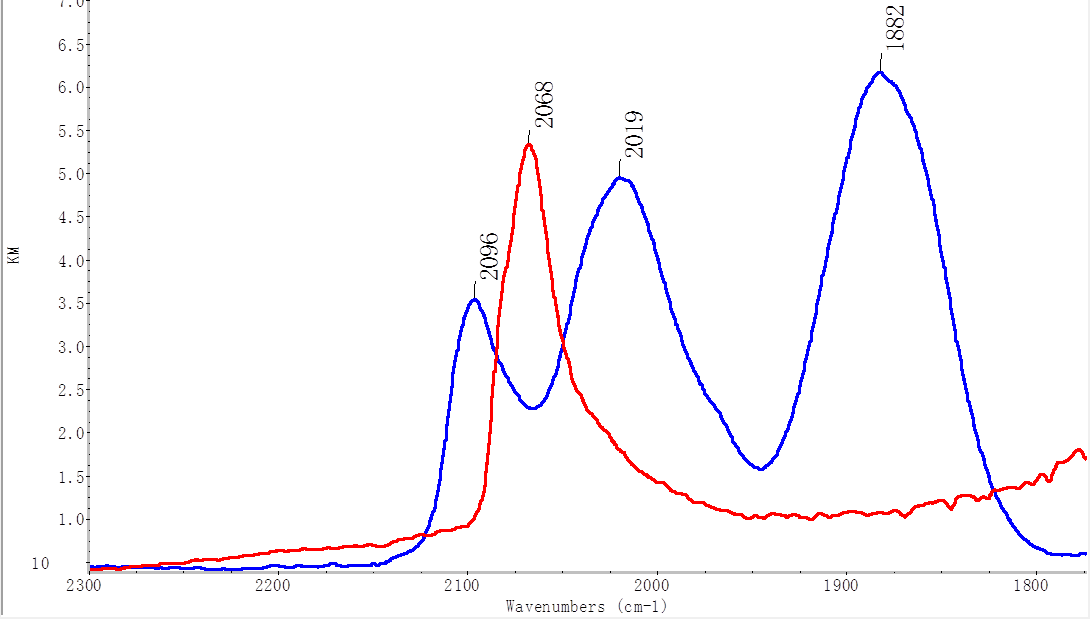
By varying the pH and pretreatment conditions for the 0.05% Pt/MgAl2O4, we were able to disperse the Pt as single atoms and subnanometer clusters. Figure 2 shows the DRIFTS spectra of CO adsorbed on this catalyst (blue) and the CO peaks show a very different pattern than those observed for nanoparticles (red). Preliminary assignments based chemisorption and assign those peaks to Pt(CO)3 with a Cs symmetry. Aberration-corrected transmission electron microscopy is currently pending (samples sent to Pacific Northwest National Laboratory where the PI has a current active proposal) on the catalysts with single atoms and small clusters to confirm the size.
Figure 2: DRIFTS spectra of CO adsorbed on 0.05%Pt/MgAl2O4 catalysts prepared by SEA where the pretreatment conditions were varied to achieve nanoparticles (red) or single atoms + subnanometer clusters (blue). The CO peaks on single atoms show a very different pattern than those observed for nanoparticles (red). Both spectra were collected after saturation while dosing with 1% CO.
The Pt single atoms and subnanometer clusters catalyst (sample shown in Figure 2) was tested for oxidative dehydrogenation of ethane. Under conditions of strict kinetic control (absence of heat and mass transfer limitations was confirmed using the Koros-Nowak test using different inter- and intra-particle dilutions), our preliminary results show a higher selectivity to ethylene on the single atoms and subnanometer clusters than large nanoparticles (large nanoparticles showed no selectivity to C2H4). However, the selectivity on Pt single atoms and subnanometer clusters was surprisingly low under the reaction conditions we tested (maximum selectivity to C2H4 was 8%). We are currently investigating whether the hypothesis that an ensemble of 2 or more Pt atoms is necessary to cleave the C-C bond by adsorbing C2H4 (intermediate for ethane dehydrogenation) in a di-sigma bonding configuration breaks down on single atoms. In other words, does the reaction proceed by a very different pathway on Pt single atoms where C2H4 and O co-adsorb on the Pt single atoms and the O can facilitate C-C cleavage? This mechanism would be very intriguing and we would be the first group to show it. However, the stability of the Pt single atoms could also be an issue and we are currently in the process of characterizing the Pt single atoms sample after reaction to check whether there was sintering under reaction conditions which results in this lower than expected selectivity to C2H4.
Our preliminary results also show that the C2H6 partial pressure could be very important in determining the selectivity for oxidative dehydrogenation of ethane. Current work is focused on characterizing the samples containing single atoms and subnanometer clusters using aberration corrected STEM and EXAFS to optimize the synthesis conditions. Additionally, we are also investigating the effect of C2H6 and O2 partial pressures on the reaction kinetics and selectivity to C2H4 as well as the stability of the catalysts under reaction conditions.