Reports: DNI555094-DNI5: In-Situ SERS Study of Au-Catalyzed Oxidation of Propene Using Multifunctional Microstructured Optical Fiber Platform
Fei Tian, PhD, Stevens Institute of Technology
Selective oxidation of hydrocarbon is one of the most critical petroleum-relevant chemical processes. Hydrogen peroxide is a desirable oxidizing agent for selective oxidation of hydrocarbons over Au. Understanding the reaction intermediates of H2O2 decomposition over Au nanoparticles (NPs) provides a great opportunity for the sustainable and green chemical production. However, the direct observation of intermediate species has been a daunting challenge, leading to the sketchy and even contradictory information in documented studies. We have discovered direct evidence of new structure of atomic oxygen adsorption on gold and OOH as reaction intermediates in H2O2 decomposition on Au under alkaline condition using ultra-sensitive surface enhanced Raman spectroscopic (SERS) measurements along with density functional theory (DFT) calculations. Large Au NPs with enhanced stability and sustained SERS sensitivity after thermal-removal of the surface ligands is the key enabler as both in-situ ultra-sensitive SERS-active probe and catalytic-active substrate for H2O2 decomposition.
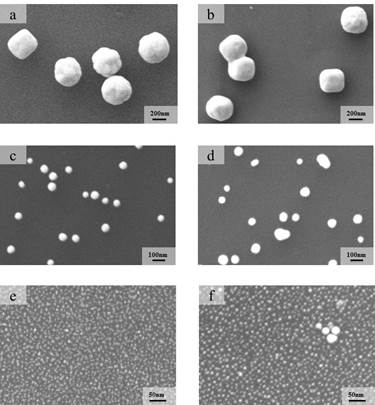
The size of Au nanoparticles and their distribution on the surface of SiO2 were analyzed in Figure 1. The ratio of the surface of Au nanoparticles to the surface of the SiO2 support remained constant. In order to remove the citrate ligands and produce metallic Au nanoparticles, the initial Au/SiO2 samples were treated under flowing N2 at 250 °C for 2 h. After the heat treatment, only the 400 nm Au particles remained largely unchanged.
Figure 1. SEM images of Au nanoparticles supported on SiO2 before (left) and after (right) thermal treatment: (a,b) 400 nm, (c,d) 50 nm and (e,f) 5 nm.
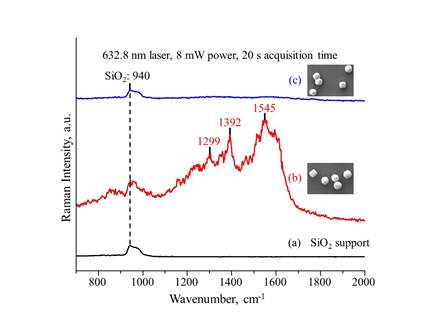
Raman spectrum for the 400 nm Au particles on SiO2 exhibited three new bands at 1299, 1392 and 1545 cm-1 (Figure 2), characteristic of the citrate ligands on Au surface. After thermal treatment, the Raman bands for the citrate ligands were no longer observed, confirming the removal of these ligands.
Figure 2. Raman spectra of (a) SiO2 support, (b) SiO2-supported Au nanoparticles prior to thermal treatment and (c) SiO2-supported 400 nm Au nanoparticles after thermal treatment.
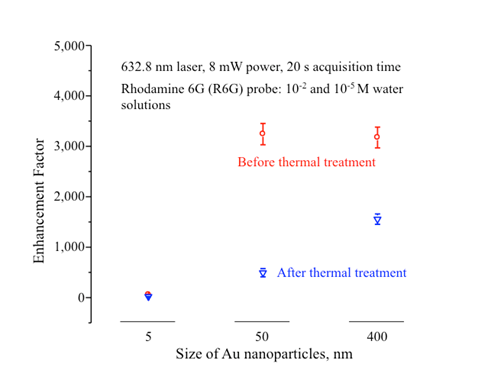
Figure 3 compares the enhancement factor (EF) calculated from Raman spectra for water solutions of R6G obtained on Au of 3 sizes before and after thermal treatment, showing that after thermal treatment the 400 nm Au particle shows the highest EF.
The thermal treatment was effective in cleaning the surface of Au nanoparticles and meanwhile allowed these 400 nm Au partiles to remain largely monodisperse and maintain reasonable SERS EFs. Therefore, the 400 nm Au particles were particularly well suited for detecting reaction intermediates on Au catalytic surfaces with Raman.
Figure 3. Raman EFs for Au nanoparticles.
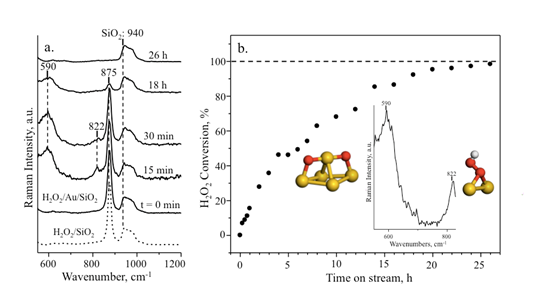
Figure 4. (a) Raman spectra and (b) titration measurements after adding H2O2 (15 wt.% in water at pH 9).
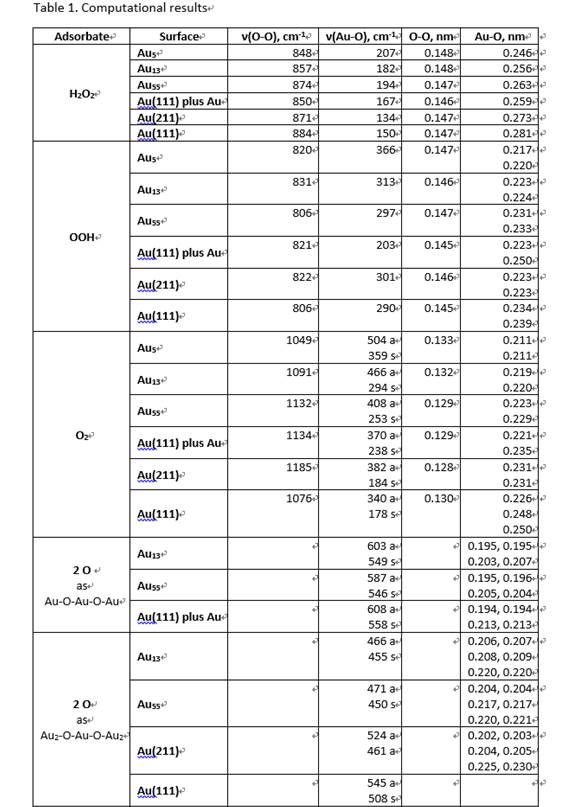
As soon as H2O2 was added, the spectrum exhibited a characteristic band at 875 cm-1 (Figure 4a), which is assigned to v(O-O) stretching vibration of H2O2. After 15 min, two new bands emerged at 590 and 822 cm-1. After 26 h, the bands at 590, 822 and 875 cm-1 disappeared. The titration results show a similar trend for the H2O2 conversion. Our DFT calculations with vibrational analyses are listed in Table 1. The band at 590 cm-1 matches reasonably with the symmetric vibration of Au-O-Au-O-Au addatom structure with a calculated value of 558 cm-1. This is a new structure identified for oxygen adsorption on Au. The band at 822 cm-1 matches well with the v(O-O) of OOH specie on Au.
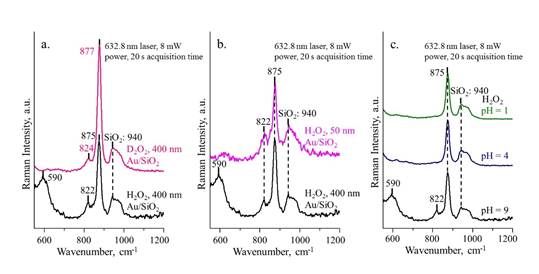
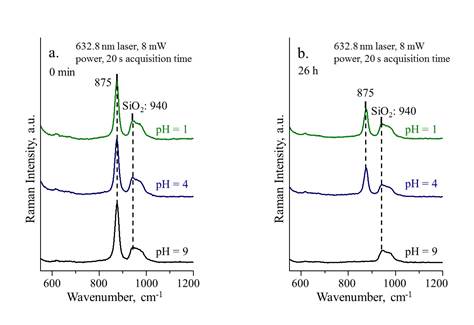
Figure 5a compares the SERS spectra of H2O2 and D2O2 decomposition obtained at 15 min. The shift of 822 cm-1 to 824 cm-1 for D2O2 confirms the OOH specie on Au. Since solution pH plays a key role in H2O2 stability, the effect of pH on the H2O2 decomposition is also studied. While the peaks of 590 and 822 cm-1 were observed for pH 9, there were no such peaks for pH 1 and 4, suggesting that acidic condition is in favor of retaining H2O2 whereas alkaline condition promotes its decomposition. Figure 6 shows the in-situ SERS spectra for H2O2 decomposition at 0 and 26 h for the three pH values. After 26 h, no peak was observed at 875 cm-1 for pH 9, while it¡¯s significant for pH 1 and 4, consistent with the titration measurement.
Figure 5. In situ Raman spectra for (a) H2O2 and D2O2, (b) three sizes of Au nanoparticles and (c) pH 1,4 and 9 of H2O2 solution.
Figure 6. SERS spectra of H2O2 with different pH at (a) 0 min and (b) 26 h.
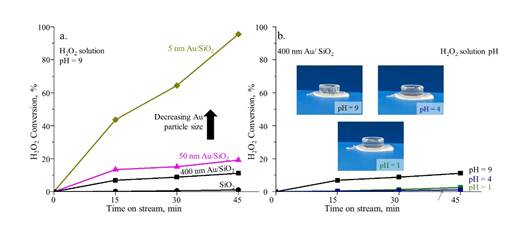
To examine the effect of particle size on the H2O2 conversion, parallel titration experiments were carried out for three sizes of Au NPs (Figure 7a). Although the total surface area of Au NPs was the same, there was a higher H2O2 decomposition rate as the size of Au NPs decreased. Figure 7b shows a clear dependence of the reaction rate on pH value of the H2O2 solution. While almost 11.1% conversion was observed for the H2O2 decomposition at pH 9, the conversion was negligible for pH 1 and 4 after 45 min. The insets of Figure 7b show no bubble under pH 1 and 4, in stark contrast to that under pH 9 with turbulent bubbling during the reaction.
Figure 7. Dependence of H2O2 conversion on (a) the size of Au nanoparticles supported on SiO2 and (b)pH of H2O2.
Through experimental and theoretical investigations, we have successfully identified a new structure of atomic oxygen adsorption on gold and OOH as reaction intermediates in H2O2 decomposition. The participated students have gained abundant knowledge and been provided opportunity to disseminate the discovery in presentation at SPIE Defense+Commercial Sensing and poster at The CATALYSIS SOCIETY of Metropolitan New York, 2016 Annual Symposium, where ACS PRF was acknowledged. We are preparing a manuscript for a top journal on this work. The project enables the PI to explore new directions, gain new collaboration, insight and expansion into the catalysis community. For future work, we are integrating a multifunctional nanostructured anodized aluminum oxide substrate for in-situ high temperature study of propene oxidation on Au using SERS.