Reports: ND753971-ND7: Interfacial Free Radical Polymerization of Thin Films
Kevin A. Cavicchi, University of Akron
Overview: The objective of this research is investigate interfacial polymerization to produce new polymer materials with properties/structures not possible by a conventional bulk or homogeneous solution polymerization.
Two types of interfacial polymerization are described. The first is the interfacial free radical polymerization of an alternating polymer at an oil/water interface to produce thin films with potential application in membrane separations. The second is the interfacial crosslinking of silicone resins to form bilayer bending actuators.
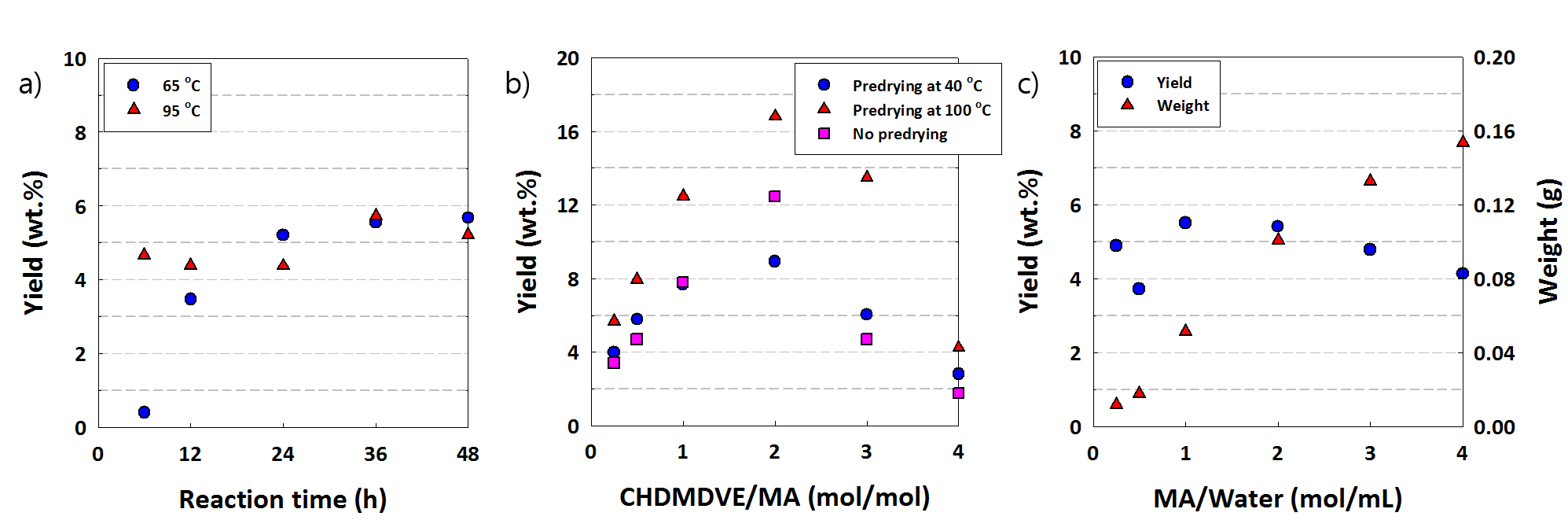
Research Highlights: In the first year of research it was established that maleic acid (MA) and 1,4-cyclohexanedimethyanol divinyl ether (CHDMDVE) are able to polymerize at a toluene-water interface to produce a thin film. However, two issues were identified with the polymerization. First, the reaction times needed to produce films were long. Second, the overall mass yield of polymer film was low and a significant amount of polymer could be extracted by swelling the film in a good solvent. To address this second issue, a protocol was developed to work-up the films, where after polymerization the solvents were removed, the recovered film was dried under vacuum at 40°C, soaked in acetone for one day, and then re-dried in the vacuum oven at 40°C to measure the mass or yield of crosslinked polymer that was formed. Figure 1a shows a plot of the yield of polymer (ratio of the final mass of polymer to the initial monomer mass) for two different reaction temperatures. The higher temperature gives a faster reaction, but a limiting yield of crosslinked film is reached. This indicates that at some extent of film formation the film prevents the transport of monomer or initiator preventing further growth of the film. To attempt to increase the conversion of crosslinked film the ratio of CHDMDVE:MA was varied and the results are shown in Figure 1b. At higher crosslinker concentrations the yield decreases with increasing crosslinker concentration. This is attributed to the formation of more linear chains, which can be extracted after polymerization. A maximum in film yield is observed at an intermediate CHDMDVE:MA ratio. The current hypothesis attributes this to two possible factors. First, the formation of a less crosslinked network could be swollen with more monomer to produce a thicker film. Second, effects from the higher monomer concentration at the interface could affect the initial film formation affecting the final conversion. To investigate the second factor we investigated the effect of monomer concentration at constant CHDMDVE:MA ratio of 0.5:1. As shown in Figure 1c, increasing the monomer concentration also increases the weight of film produced, indicating the local concentration of monomer affects the overall film formation, but interestingly the yield of film is not strongly dependent on concentration. This result implies that to maximize the efficiency of film formation, the surface area to volume ratio of the film to solvent should be increased. This will be investigated in the final year of this research. One other point is shown in Figure 1b where the films were either dried at 100°C or not dried before the second solvent extraction step in the workup. Drying at higher temperature significantly increases the yield of the polymer film. This indicates that additional crosslinking may occur after removal of the solvent, which could be further utilized to increase the film yield.
Figure 1. (a) yield vs. monomer ratio. (b) yield vs. reaction time. (c) yield/weight vs. monomer concentration.
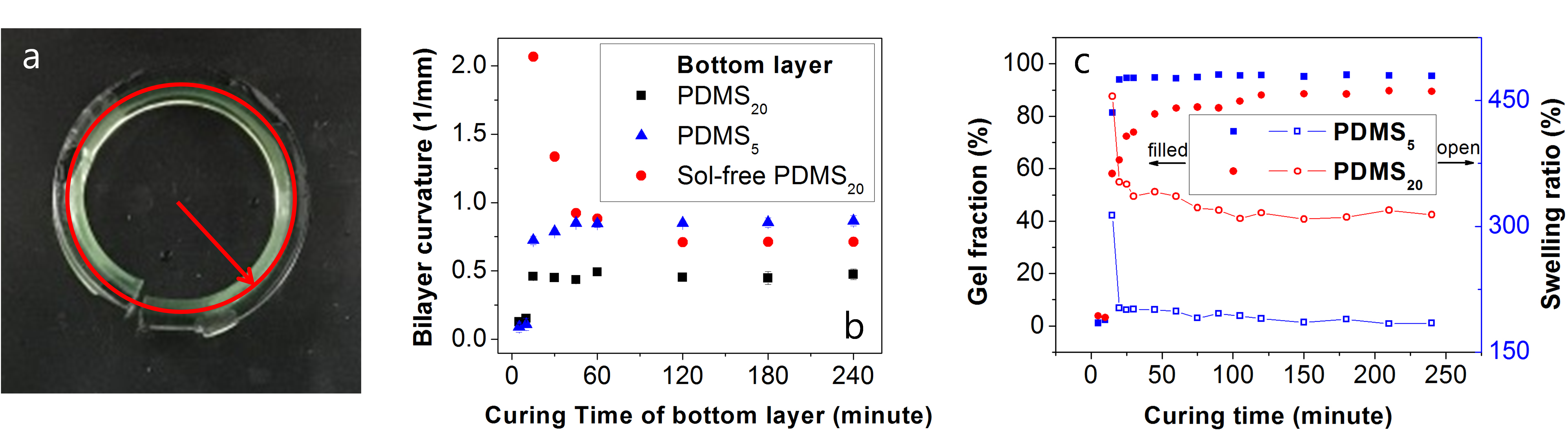
In a second investigation the crosslinking of PDMS bilayers was investigated using a two part platinum cured silicone where the crosslink density is varied by varying the weight ratio of base resin to curing agent. Two formulations were investigated with base:curing agent ratios of 5:1 and 20:1 to produce films with high and low crosslink density, respectively. Bilayer films were made by first pouring either the 5:1 or 20:1 formulation into a mold and crosslinked at 80°C for time t, and then the second formulation was poured on top of the first layer and crosslinked for 2 hours at 80°C. When immersed in solvent bending is observed, as shown in Figure 2a, driven by the minimization of the stress generated by the differential swelling of the PDMS layers. Figure 2b shows the curvature vs. crosslinking time where either the 5:1 or the 20:1 formulation was crosslinked first. Higher curvature is obtained when 5:1 formulation is crosslinked first. This is attributed to the lower gel fraction of the 20:1 crosslinked PDMS at 80°C (Figure 2c). When the 20:1 formulation is crosslinked first, it is postulated that the remaining sol fraction diffuses into the second layer broadening the interface. To test this, the sol fraction of the 20:1 crosslinked sample was extracted prior to the addition of the second 5:1 layer. The amount of resin used for the second layer was adjusted to match the weight of the extracted bottom layer, which shrinks at low gel fraction. At long crosslink times this produces an intermediate bending curvature. The high curvature at short crosslink times is due to the lower crosslink density of the 20:1 layer and the lower overall thickness of the bilayer.
Figure 2. (a) Swollen bilayer. (b) curvature vs. curing time. (c) gel fraction/swelling ratio vs. curing time.
Significance of the Research: The introduction of new polymerization procedures to produce films by interfacial polymerization expands the backbone chemistries available, which is useful to tailor properties including the stability and functionality. The optimized process for fabricating bilayer actuators using commercially available materials will be useful for the development of new materials for sensing and programmed motion.
Impact of PRF Funding: Three graduate students (1 MS and 2 PhD) have participated in this work. The funding supported the materials and supplies for the MS student’s thesis. This MS student graduated in summer 2016 and entered a PhD program at Central Florida University. The funding has supported the research and stipends of the two PhD students. The funding has allowed the PIs research group advance into two new areas of research, interfacial free radical polymerization and bilayer polymer bending actuators.