Reports: DNI1055789-DNI10: Interfacial Phenomena of Versatile Janus Particles at Fluid-Adjoining Interfaces and Their Potential Applications in the Petroleum Industry
Younjin Min, PhD, University of Akron
Introduction
Janus colloidal particles with anisotropic shape and/or surface properties have attracted significant attention due to their unique morphologies, controllable molecular recognition, and self-assembling processes. Although various interesting aspects of their collective behaviors at interfaces have been studied, there are a lot of unknowns about their kinetics and dynamics of assembly into unique nanostructures and physicochemical mechanisms governing the phase behavior and stability of such nano-assemblies. This research is aimed at obtaining an understanding of interparticle interactions and interactions between a particle and the surrounding liquid for Janus nanoparticles; how these interactions govern the kinetics and thermodynamics of nanoparticulate assembly as well as phase behavior and stability of such assemblies.
Results
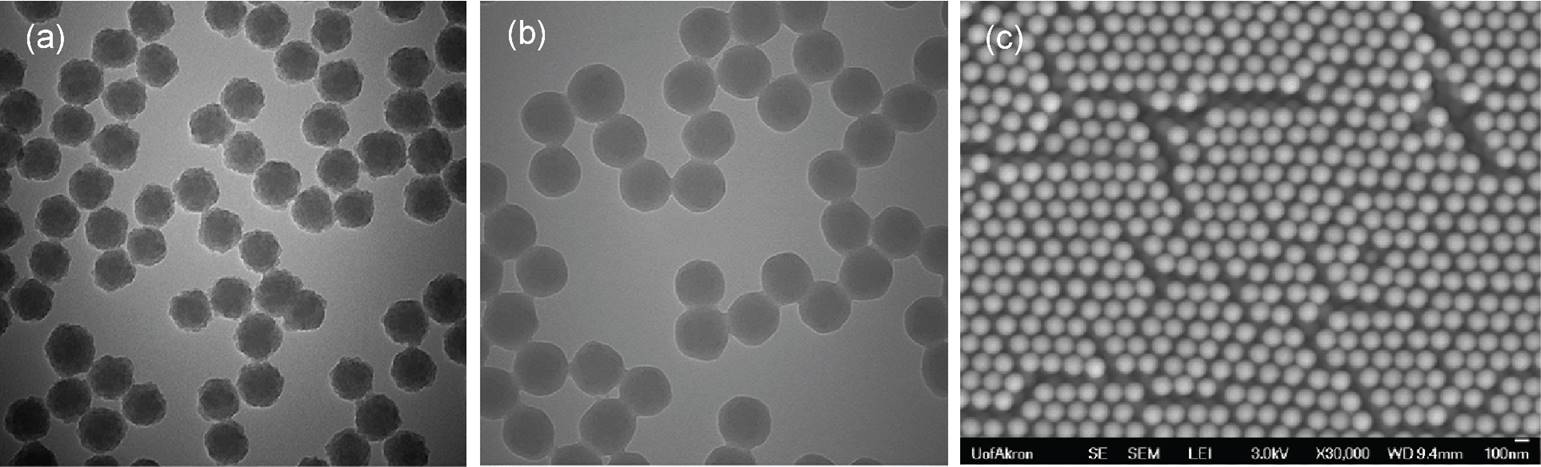
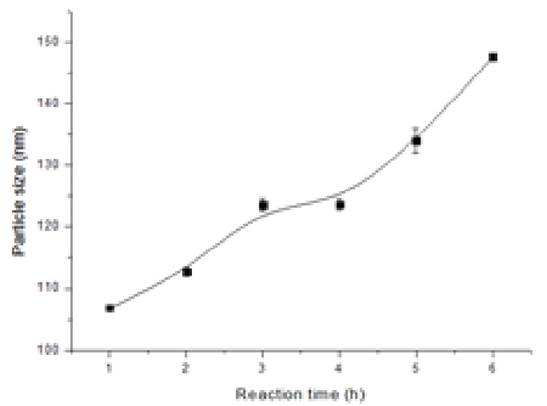
The production of monodisperse and well-defined Janus nanoparticles is a pre-requirement to successfully carry out the proposed studies. Hence, we initially focused on the production of high-quality Janus particles. Here, silica nanoparticles are selected as the nanoparticulate precursors for Janus nanoparticles because of their low cost and environmentally friendliness. We have tried various synthesis approaches for the production of ideal base silica nanoparticles, and had the best success with seed regrowth approach for nanoparticles smaller than 300 nm and with tetraethyl orthosilicate (TEOS) step addition-dilution approach for nanoparticles larger than 300 nm. Figure 1 shows TEM/SEM micrographs of the silica seeds before and after regrowth step of varying degree. After the Stӧber regrowth step, SiO2 nanoparticles formed were very monodisperse and perfectly spherical (Figure 1c). The systematic time-resolved studies revealed that the particle size was roughly linearly dependent on the regrowth reaction time in this method (Figure 2).
Figure 1. TEM images of (a) silica seed nanoparticles of 24.1±0.6 nm size, (b) silica nanoparticles grown on silica seeds after a short regrowth time. (c) SEM image of silica nanoparticles grown on silica seeds after a long regrowth time.
Figure 2. Effect of regrowth reaction time on the particle size.
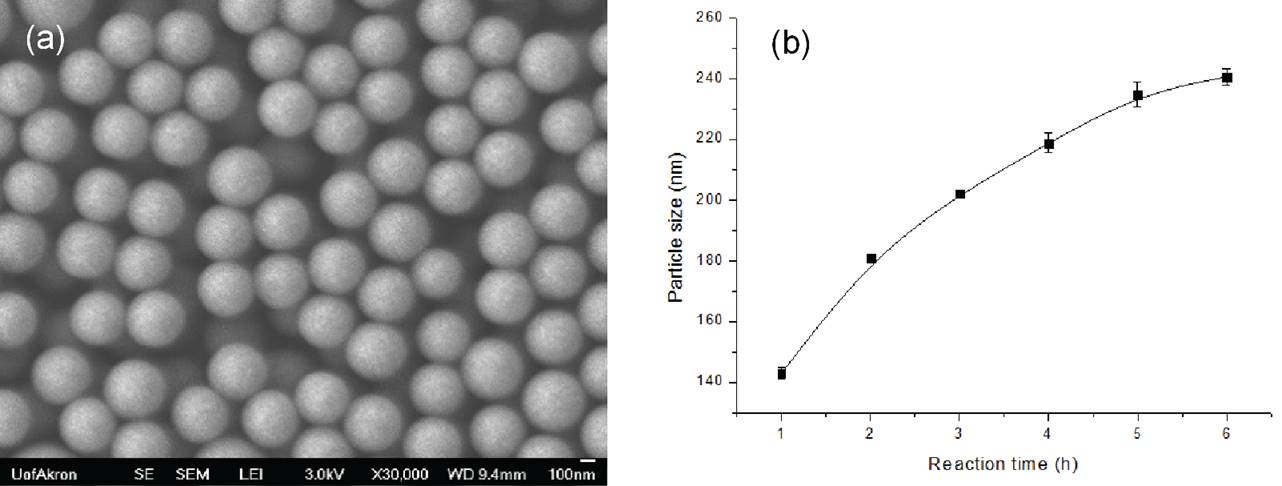
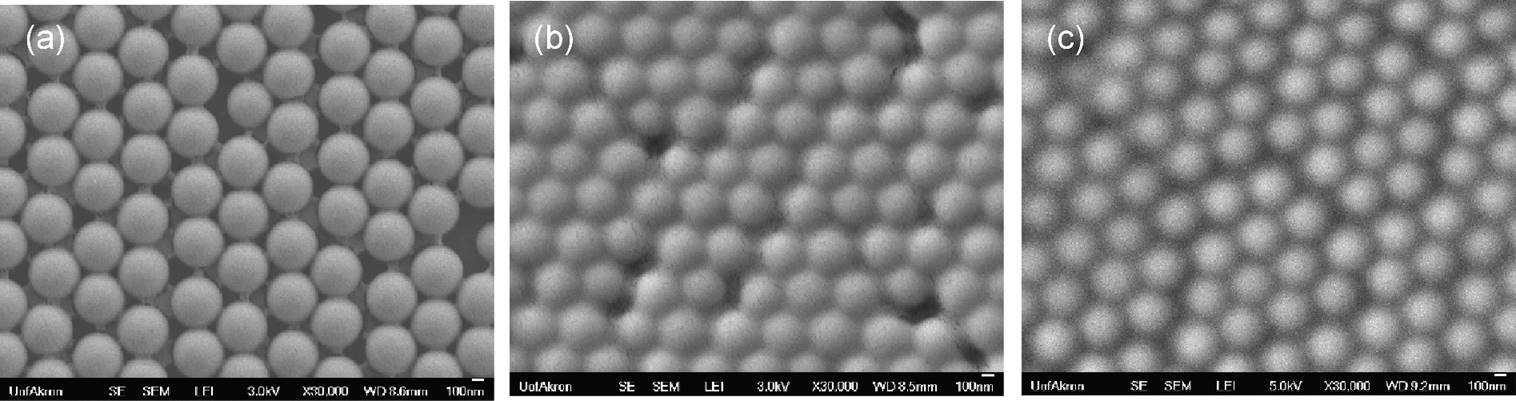
TEOS step addition-dilution approach also yielded spherical nanoparticles with a small variation in size (Figure 3a). In this approach, the growth in particle size was sublinear with respect to reaction time (Figure 3b), indicating a different mechanism of reaction.
Figure 3. (a) Representative SEM image of silica nanoparticles and (b) Effect of reaction time on the particle size distribution prepared by TEOS step addition-dilution approach.
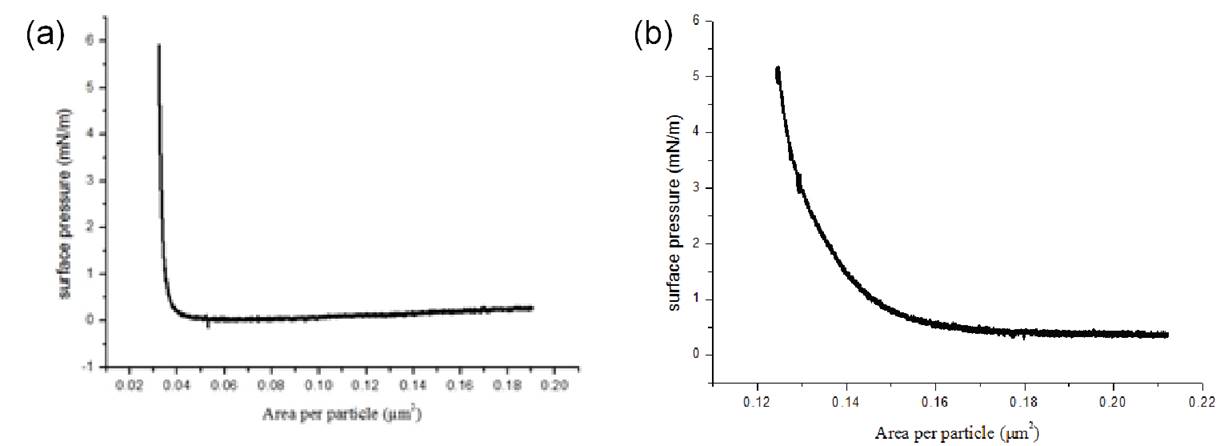
After preparing high-quality SiO2 nanoparticles (NP), we focused on the selective functionalization of these nanoparticles to produce Janus particles. To control the degree of coating/functionalization, we first formed monolayers using silica nanoparticles via Langmuir deposition technique. We found that concentration of SiO2 NPs influences the lateral interparticle forces and dynamics of their organization. For instance, at a concentration of 16 mg/mL, surface pressure-area (π-A) isotherms experienced no repulsive force until hard wall is reached (Figure 4 (a)). On the other hand, at a concentration of 60 mg/mL, π-A isotherms experienced almost an exponential repulsive force (Figure 4b). By comparing structure of Langmuir deposited monolayers at different surface pressures, we identified that the surface pressure in the range of 1 to 5 mN/m yielded the closely-packed SiO2 NP monolayers. The reason we sought to control the packing of SiO2 is that the next step is a conformal deposition approach the nanoparticulate coverage of which depends on the nanoparticulate spacing and available projection area for deposition.
Figure 4. Representative surface pressure-area (π-A) isotherms at nanoparticle concentrations of (a) 16 mg/mL and (b) 60 mg/mL.
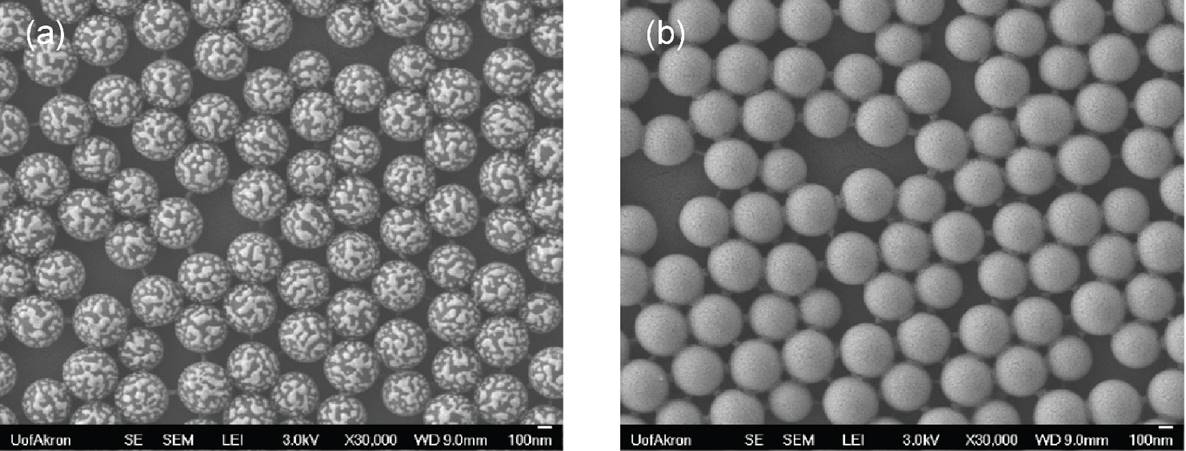
Aside from nanoparticulate spacing, we utilized three different approaches to control the lipophilic-hydrophilic balance on Janus particles. To this end, poly(methyl methacrylate), PMMA, was used as masking agent for coatings. While the deposition of PMMA on bare silica nanoparticles were poor, resulting in patchy Janus nanoparticles, the deposition on plasma treatment led to continuous coverage (Figure 5). This is presumably due to the mismatch in the interfacial energy of bare silica nanoparticles and PMMA. We were able to control the lipophilic-hydrophilic balance of Janus nanoparticles by adjusting the concentration of PMMA (Figure 6). As expected, high concentrations of PMMA gave rise to thicker coating layers and further higher area ratio of lipophilic to hydrophilic.
Figure 5. SEM images of PMMA covered silica nanoparticles (a) without and (b) with plasma treatment.
Figure 6. SEM images of PMMA covered silica nanoparticles prepared from different PMMA concentrations of (a) 1%; (b) 3%; and (c) 5% solutions.
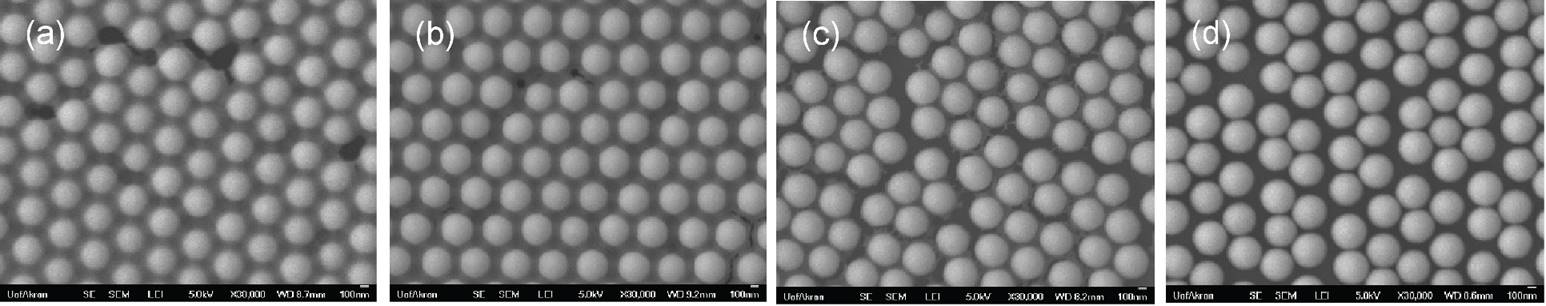
The PMMA covered silica monolayers were then introduced in a plasma chamber under vacuum, air, and O2 stream. The exposed surface areas in silica particles were precisely controllable under plasma treatment in the presence of O2 stream according to treatment time as shown in Figure 7.
Figure 7. SEM images of PMMA covered silica nanoparticles prepared after plasma treatment under O2 stream for (a) 1 minute; (b) 3 minutes; (c) 4 minutes; and (d) 6 minutes.
The exposed silica surfaces were modified by three different types of organosilane molecules such as trimethylchlorosilane, pentyltrichlorosilane and phenyltrichlorosilane in aid of a chemical vapor deposition method. In order to confirm a successful surface modification, a contact angle measurement was carried out. In a given reaction time range of 0.5-8 hours, PTS showed the most hydrophobic nature most likely due to its long alkyl chain as well as relatively tight packing density when it forms self-assembled monolayers on the surface.
Future work
Now, we have completed some aspects of Task 1, aiming at establishing procedure for the production of well-defined Janus nanoparticles, and Task 2, investigating the lateral interactions of Janus particles using Langmuir-Blodgett technique. Our future studies will aim at obtaining a complete comprehension of the phase behavior of such a water–oil–Janus nanoparticle system as a function of composition, temperature, pressure, and the design parameters of Janus nanoparticles. Also, we will utilize the surface force apparatus (SFA) technique to directly measure the nanoscale interactions between two oil–water interfaces containing Janus particles and interfacial coalescence behavior. Finally, we will study the dynamics and rate of Pickering emulsion formation using on Quartz Crystal Microbalance with Dissipation.