Reports: DNI754462-DNI7: Fundamental Studies of Material Properties of Chemically Cross-Linked Gels Concentrated above the Overlap Concentration
Kelly Schultz, PhD, Lehigh University
Cross-linked gels play a significant role in enhanced oil recovery (EOR) by decreasing permeability in high permeability zones near naturally fractured carbonates that require water shutoff but cannot be permanently plugged. The objective of this work is to study the fundamental principles that govern material properties of cross-linked polymeric gels concentrated above the overlap concentration, c*. These history-dependent systems are monitored during gelation. This work will establish a quantitative framework to understand how polymeric overlap and entanglements within macromer solutions change the gelation reaction and influence final material properties in chain and step-growth systems. Rheological measurements quantify dynamic material properties during gelation. Multiple particle tracking microrheology (MPT) measures the properties of the weak incipient gel as cross-links form pinpointing the sol-gel transition. Microrheology uses optical techniques to capture data enabling the identification and quantification of spatial heterogeneity. Techniques for measurement of heterogeneity are developed in a colloidal gel that displays heterogeneity over an observable window of 250×250 microns. Bulk rheological measurements capture properties of gels as the stiffness is increased above the measurable limit of microrheology.
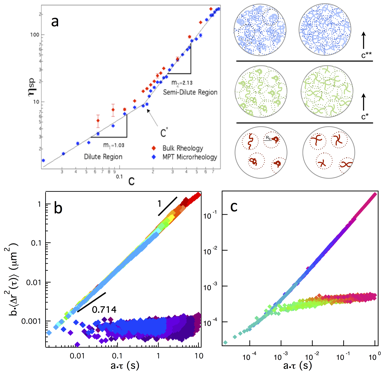
Determination of overlap concentration of the backbone molecule. This work is motivated by the need for manipulation of polymeric cross-linked gel properties to address unique situations faced on site during EOR. In order to understand the function of these gels, we must first understand how precursor solution polymeric interactions change the evolving material properties during gelation. c* is determined by measuring the viscosity of the polymer solution. Polymeric solutions have three regimes in the concentration-viscosity curve, the dilute, semi-dilute and entangled. The first two regimes are illustrated in Figure 1a with a viscosity curve on the left and illustrations on the right show the three regimes for multi-arm poly(ethylene glycol) polymers that will be the backbone of our gels. c* determined from bulk, microrheological measurements and theoretical calculations is 14.3 wt% for the backbone molecule. The goal of this work is to establish a fundamental understanding of how the interplay between entanglement and cross-linking changes the polymeric assembly and structure of the network.
Polymeric gel systems. We use rheological characterization and fundamental principles of polymer physics to determine the gel point, time scale of gelation and heterogeneity of the microstructure as the material forms. This work studies two mechanisms for gelation, chain and step-growth. Previous studies have compared these mechanisms below the overlap concentration but the efficiency of gelation when polymers interact will be quantified. The step-growth gel studied is a four-arm star PEG-acrylate (Mn 20 000 g/mol) cross-linked by photopolymerization with a linear PEG-dithiol (Mn 1 500). The chain-growth reaction is a four-arm star PEG-maleimide (Mn 20 000 g/mol) that self assembles with a linear PEG-dithiol (Mn 1 500 g/mol). The backbone and cross-linker for each gel is the same size, therefore, any variation in structure is due to the mechanism of gelation.
Figure 1. Changes in material properties due to polymeric overlap. (a) Concentration-viscosity curve measured for four-arm star PEG molecules identifies c* (b) Time-cure superposition of 3 wt% (b) step-growth PEG-acrylate and (c) chain-growth PEG-maleimide gels. This analysis determines that the scaffolds form different structure due to the cross-linking reaction.
Identifying scaffold critical properties and structure during gelation. Time-cure superposition (TCS) is used to analyze both of these gel scaffolds at concentrations above and below the overlap concentration. The critical relaxation exponent indicates the structure of the network and the ability for the network to store and dissipate energy. The concentration measured below c* is 3 wt% four-arm star PEG with a ratio of backbone reactive sites to cross-linker reactive sites of 0.65. The PEG-acrylate scaffold is exposed to light then the reaction is stopped and properties are measured as a function of UV exposure time. The PEG-maleimide gel self-assembles, therefore, the reaction is measured through time. Using TCS, we find the critical relaxation exponent does change due to the type of cross-linking. At 3 wt% the value of n for PEG-acrylate and PEG-maleimide is 0.70±0.01 and 0.43±0.02, respectively. Sol and gel master curves are shown in Figure 1b and c. The PEG-acrylate forms a loosely cross-linked gel network that dissipates energy and the PEG-maleimide forms a tightly cross-linked network that stores energy. Additionally, the PEG-acrylate is measured at a concentration above c*, 16 wt%. The value of n does change when the material is concentrated and is 0.61±0.01. This is still a loosely cross-linked architecture but there is greater ability to store energy at higher polymer concentrations.
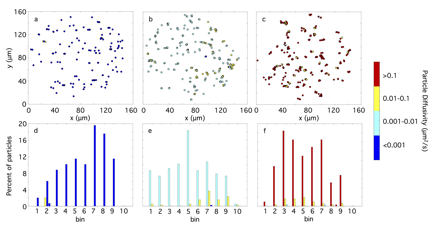
Analysis of heterogeneity in model colloidal gel system. Hydrogenated castor oil (HCO) is a colloidal gel that has spatially heterogeneity during gel transitions. This gel is degraded by submerging a sample into water, and the colloids contract when contacted with a surfactant. MPT is used to quantify heterogeneity during the critical transition of HCO gels. Traditionally, MPT is only applicable to homogenous materials. Here, we develop techniques to quantify material properties of different microenvironments in heterogeneous materials. Variances in individual probe particle van Hove correlation functions are compared using an F-test with a 95% confidence interval, separating particles probing different microenvironments, Figure 2. Material properties are calculated for each separate microenvironment. Heterogeneities during the critical transition are quantified and diffusivities of microenvironments are reported. With the work presented, MPT can be used to accurately quantify material properties of individual microenvironments for a heterogeneous material.
Figure 2. Rheological (a-c) and spatial (d-f) heterogeneity of probe microenvironments for the gelsol transition at a reduced time, tr of (a, d) -0.90, (b, e) 0, and (c, f) 0.82.
Future work. Current work on this project is measuring the material properties of the PEG-maleimide network above c*, at 16 wt%. This work will also include measurements of both scaffolds directly below c*, at 10 wt%. These measurements will determine whether simply concentrating the polymeric precursor solutions leads to different scaffold structures or if this is due to the interaction of molecules above c*. These measurements will lead to a better understanding of how polymer concentration and interactions change gel scaffold structure.