Reports: UR654397-UR6: Spectroscopic Characterization of Acrolein in Its Lowest Triplet (n,pi*) State
Stephen Drucker, University of Wisconsin (Eau Claire)
Acrolein (CH2=CH–CH=O) is the smallest alpha,beta-unsaturated carbonyl molecule and serves as a prototype for investigating the photochemical properties of larger analogs. Much of this photochemistry is mediated by triplet excited species, including the T1(n,pi*) state. In this project, we will obtain experimental information about the structure and dynamics of acrolein in its T1(n,pi*) state. To accomplish this goal, we are planning to record the T1←S0 cavity ringdown (CRD) spectrum of acrolein and deuterated isotopologues in a jet-cooled planar expansion. We will analyze the 0−0 band contours to obtain sets of inertial constants which will in turn establish the equilibrium geometry of the T1(n,pi*) state.
During the past year of the grant, the PI and undergraduate collaborators worked on modifications of our jet-cooling apparatus to convert the current pinhole expansion into a planar-jet expansion. The planar expansion will afford a much longer absorption pathlength than the pinhole and will improve the signal-to-noise ratio in the planned CRD absorption studies of acrolein.
While the jet-cooling system was undergoing modification, we focused on room-temperature spectroscopic studies of the 2-cylohexen-1-one molecule (2CHO). This molecule has the same conjugated enone chromophore as acrolein. Also the 2CHO molecule, like acrolein, has conformational flexibility that promotes nonradiative processes in the (n,pi*) excited states. These processes are responsible for the very short observed excited-state lifetimes of both CHO and acrolein.
Computational investigations of acrolein and 2CHO will potentially offer the deepest insight regarding the mechanisms of their nonradiative decay; our PRF-supported experimental work is aimed at benchmarking aspects of excited-state computational techniques. Economical methods such as CISD and TDDFT are able to illuminate broad photophysical features of these molecules, but accurate details of the potential-surface topologies are unavailable from these methods because of their limited treatment of electron correlation. The most promising computational approaches are ab initio techniques able to treat both static and dynamic electron correlation efficiently. Among such methods, EOM-EE-CCSD currently has the most favorable cost/accuracy ratio for medium-sized molecules such as 2CHO.
Our planned studies of acrolein include spectroscopic determination of triplet-state vibrational frequencies, which will be compared to EOM-EE-CCSD computed results. As a prelude to this work, and while we finalize construction of our jet-cooled apparatus, we have recorded the S1(n,pi*) ← S0 CRD spectrum of 2CHO in a static cell. Jet cooling was not required for the 2CHO investigation, in contrast to the planned acrolein work. This is because the targeted T1(n,pi*) ← S0 vibronic transitions of acrolein are coincident with interfering S1(n,pi*) ← S0 hot bands, whereas the S1(n,pi*) ← S0 band system of 2CHO is spectrally isolated from other electronic transitions. We were able to measure the 2CHO spectrum readily at room temperature using our CRD apparatus. The present findings, which focus on the C=O/C=C stretch region of the S1 state, complement our earlier work [J. Phys. Chem. A 112, 38 (2008)] on the CHO S1(n,pi*) ← S0 transition near its vibronic origin.
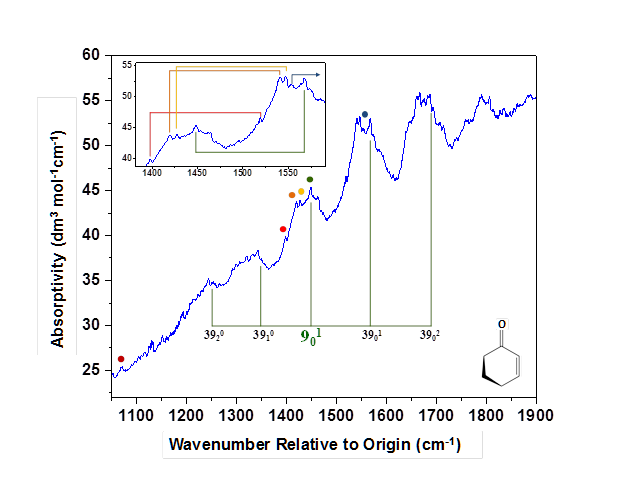
The figure below shows the room-temperature CRD spectrum of 2CHO we recorded under current PRF support. The horizontal scale reports wavenumber with respect to the S1(n,pi*) ← S0 origin band (383.4 nm or 26081 cm-1). During the current reporting period, we completed a comprehensive vibronic analysis of the spectrum. This analysis has afforded fundamental frequencies for several vibrational modes in the S1 state, including a ring-bending vibration, C=C stretch, CO—CC stretch, and a group of four different modes involving significant C=O stretch character. The fundamental of each newly assigned mode is identified in the figure with a colored dot. The assignments are supported by observation of combination bands that form progressions built upon the parent fundamentals. The progressions correspond to the lowest-frequency vibrational mode, nu39, in both the ground and excited states. These same intense progressions in nu39 (ring twisting) were observed in the origin-band region. The nu39 progressions attached to nu9 (CO—CC stretch) are labeled explicitly as a representative example. The inset shows the 3901 combination band attached to several of the fundamental bands identified in the main spectrum.
Comparisons between computed and experimental frequencies confirm that the EOM-EE-CCSD technique is significantly more accurate than CISD or TDDFT when applied to this prototypical enone molecule. Mean absolute errors are on the order of 15 cm–1 for the EOM-EE-CCSD method and 30 cm–1 for CISD and TDDFT. This outcome suggests that one must use caution in employing economical computational methods for molecules (such as 2CHO) in which the lack of symmetry leads to extensive configuration mixing in the excited state. We have also found that even when using a highly correlated technique such as EOM-EE-CCSD to calculate excited-state properties, it is necessary to use a triple zeta-quality basis set in order to realize the high intrinsic accuracy of the method.
In planned work on the acrolein molecule, we will determine C=O and C=C stretch frequencies for the T1 state, as we have done for the S1 state of 2CHO. Jet cooling of acrolein will also allow us to determine a substitution structure for the T1 state, via analysis of the rotational contours for the parent species and deuterated isotopologues. The PI has recruited a new student, Noel Weber, to prepare the deuterated compounds. To provide appropriate mentorship to Ms. Weber, the PI is collaborating with organic chemists on the UW-Eau Claire faculty. The faculty colleagues have helped refine Ms. Weber’s synthetic skills (and those of the PI!) in preparation for upcoming acrolein work.