Reports: UR355441-UR3: Synthesis, Structural, and Spectroscopic Studies of Porphyrin Based Model Asphaltene Lanthanide and Group 11 Bimetallic Complexes: Intra- and Inter-Molecular Excimers and Exciplexes Studies in Solution and Solid State
Zerihun Assefa, North Carolina A&T State University
Introduction
The ultimate research goal of this project is to rationally select porphyrin based model complexes that will be used to simulate asphaltene aggregations. The materials involve bimetallic systems containing group 11 transition metal (in particular cyanoaurate complex) and selected lanthanide ions. The compounds are expected to exhibit excimer and exceplexes properties that may arise due to pi-staking and/or metallophillic interaction.
As a first phase of this project we have initiated synthesis of phthalate based lanthanide systems that can be inserted into the porphyrin core. In this regard, the coordination of 4,4-Biphenyl dicarboxylate ligand has been conducted using mechanochemical synthesis technique [1-2]. The ligand, biphenyl-4,4’-dicarboxylic acid, has hard Lewis base carboxylate groups at the n the para positions of the ring that allows for coordination to Lewis hard acid metals. The biphenyl group can act as a spacer if the ligand coordinates at both sites.
The ligand’s coordination to Eu3+ ions was studied by reacting it with europium triflate and europium chloride salts. The complexes were synthesized via the mechano-chemical method. Although such reactions could be achieved through the use of vibratory, ball, and planetary mills, the instrument has not arrived for this work and the synthesis was conducted on mortar and pestle grinding technique.
Methodology
All chemicals were purchased Sigma Aldrich and used without further purification. A mixture of 50.08 mg (0.084 mmol) of Eu(CF3SO3)3 and 41.56 mg (0.172 mmol) of biphenyl-4,4’-dicarboxylic acid (BPDCA) was placed into an Agate mortar and pestle and ground under air for three minutes. There was no color change noted of the powder during the grinding process.
Similar procedure was followed for the mixture of 52.32 mg (0.143 mmol) of EuCl3•6H2O and 60.11 mg (0.248 mmol) of BPDCA reaction. This powder was highly hygroscopic forming aggregate clumps.
The powder product was characterized by FT-IR and luminescence spectroscopy methods. The infrared spectra were collected on a Shimadzu IR Prestige-21 equipped with a class 2 Helium-Neon laser and 0.1mW max optical power. Luminescence spectra were collected on a PTI QM-7/SE model spectrometer equipped with: 75W xenon bulb, 928 model PMT detector, and FeliX32 fluorescence software package
Results & Discussion
FT-IR characterization of the Eu(BPDCA) complexes shows vibrational stretches at 3417, 1674, 1606, 1296 cm-1 corresponding to the νOH, νC=O, νCC, and νCO. The Eu(CF3SO3)3(BPDCA) complex showed additional stretches at 1240 and 1029 cm-1 corresponding to the νCF and νSO. The chloride hydrate complex also shows stronger hydroxyl stretching due to the hygroscopic nature of the complex along with the coordinated water molecules of the inner coordination sphere.
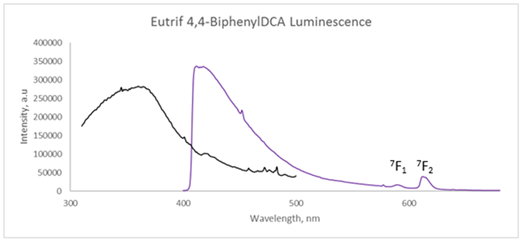
Luminescence characterization of the hydrated Eu(BPDCA)(trif) complex shown in Figure 1 depicts the excitation and emission profiles, respectively. The excitation profile shows a dominant, broad band at 360 nm when monitored at the Eu3+ emission line of 5D0 à7F2 transition. The corresponding emission profile when excited at 360 nm shows a dominant ligand emission at 450 nm and sharp, but weak Eu3+ f-f transition indicating very little coupling between the excited state of the ligand and the Eu3+ ion.
Figure 1. Luminescence spectra of Eu(BPDCA)(trif) complex
Compared to the luminescence of the Eu(BPDCA)(trif) system, the data for Eu(BPDCA)Cl3 complex has a better energy transfer phenomenon from the organic ligand to the lanthanide ion.
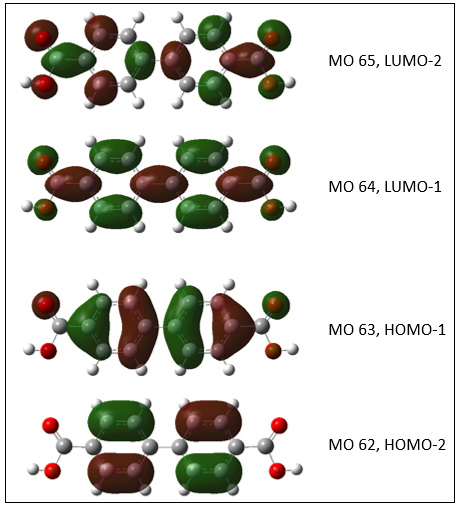
Further theoretical simulation of the system was conducted DFT calculation. Electron density distribution about the 4,4-biphenyldicarboxylic acid compound derived from the Gaussian 09 computational modeling program is shown in Figure 2. The DFT calculation was conducted using a 6-311G basis set. The figure shows the first two LUMO and HOMO molecular orbitals showing the transition with an increase in energy.
Figure 2. Electron density of BPDCA molecular orbitals
Reference
[1] Friscic, Tomislav., et al., Clean and efficient synthesis using mechanochemistry:
coordination polymers metal organic frameworks and metallodrugs, Croat. Chem. Acta. 2012,
85, 367-378.
[2] Ranu, Brindaban. Ball Milling Towards Green Synthesis: Applications, Projects, Challenges. RSC Green Chem, 2015. Print.