Reports: DNI153951-DNI1: Facile Access to Vicinal Amino-Heteroatom Functionalities from N-X Bonds
Qiu Wang, PhD, Duke University
Scientific goals: The objective of this project is to develop novel amination strategies of nitrogen-halide bonds (N-X) to achieve a general and modular approach to access vicinal amino-heteroatom-containing (e.g. 1,2-aminohalide and amino oxygen) important structural and functional motifs in catalyst ligands, chiral auxiliaries, as well as naturally occurring and biologically active molecules (Figure 1).
Figure 1. Aryne insertion as a modular and rapid access to ortho-functionalized aminoarenes
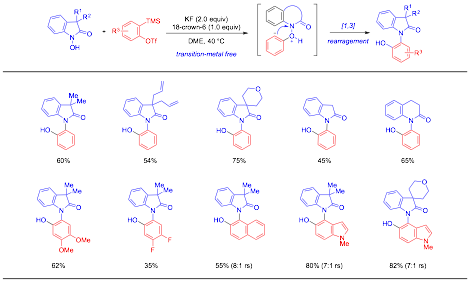
Progress: During the second year, we have made progress on exploring the reactivity of nitrogen-oxygen bonds to construct valuable ortho-amino oxygen arene motifs. We have developed a novel and efficient synthesis of ortho-aminophenols via the addition of hydroxyindolinones to arynes followed by a chemo- and regioselective [1,3]-rearrangement (Scheme 1). This transformation offers a rapid and efficient entry to diverse ortho-aminophenol scaffolds with as a good functional group tolerance under mild transition-metal-free conditions, presenting useful applications in the synthesis of sterically hindered aminophenol-containing natural products, materials, and ligands. A manuscript on this study has been recently published (Org. Lett. 2015, 17, 6130–6133).
Scheme 1. Synthesis of ortho-aminophenols by formal aryne insertion with hydroxyindolinones
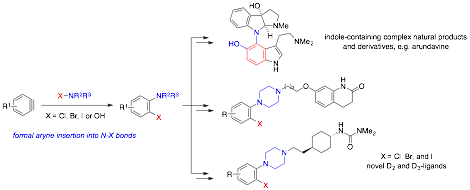
On the other hand, we have advanced our studies to demonstrate the application of aryne insertion chemistry with nitrogen-halide bonds for the preparation of functionally important amino arenes (Scheme 2), such as indole-containing natural products and novel D2/D3 ligands, especially those that are difficult or inaccessible otherwise.
Scheme 2. Rapid and modular synthesis of functionally important aminoarene skeletons.
Impact: Our research funded by ACS PRF has made significant impact: (1) it discloses diverse reactivity of nitrogen-heteroatom bonds for novel amination reactions; (2) it demonstrates synthetic utilities of new amination transformations for the rapid synthesis of biologically active ortho-functionalized aminoarenes; and (3) it provides critical trainings for both graduate and undergraduate students in their career development. Undergraduates involved in the project have presented at ACS meetings and departmental poster sessions and have continued on scientific career paths. Current research progress also laid foundation for the future work on developing diverse and general reactions using nitrogen-heteroatom bonds to construct valuable vicinal amino-heteroatom functionalities.