Reports: UNI753097-UNI7: Structure-Activity Study of Triazolium-Containing Michael Addition Polyesters
Kevin M. Miller, PhD, Murray State University
Undergraduates in our laboratory have been working on projects that encompass two broad areas of organic/polymer chemistry: ionic liquids (ILs) and poly(ionic liquid)s (PILs). The overall objective of our research program is to explore novel triazolium ILs and PILs through the correlation of structure with properties (viscosity and conductivity for ILs; thermal and mechanical stability for PILs).
Project I: Explorations in Triazolium Ionic Liquids (partial ACS-PRF support):
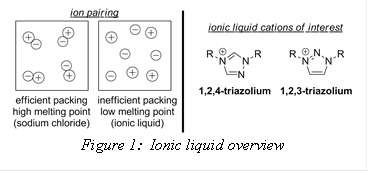
For decades, industrial chemists have been trying to find alternative, “greener” solvents in which to perform chemical reactions. Most common industrial solvents are volatile, flammable, often times toxic and difficult to recycle. During the course of exploring solutions to this growing issue, ionic liquids were identified as potential alternatives. An ionic liquid (IL) is a salt consisting of weakly coordinating ions that typically exists in the liquid state below 100 °C (Figure 1). Advantages to using ILs over commercial solvents include reduced vapor pressures, flammability and toxicity levels. Many of these materials have even been shown to exhibit recyclability. Imidazolium and phosphonium ILs (the two most researched classes) have been utilized in a variety of applications ranging from green solvent replacements to liquid electrolytes in batteries and fuel cells. As a result of the increasing interest for ILs in energy devices, our group has become involved in exploring triazolium-containing ionic ILs. Since the triazolium ring contains an additional nitrogen it has been proposed as a more “energy-rich” alternative to imidazolium.
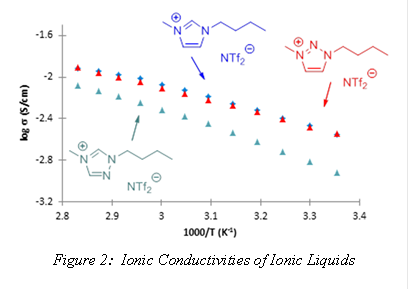
Efforts from our group over the past year have focused on the synthesis and properties of a series of 1,2,3-triazolium [NTf2] (bistrifluoromethanesulfonate) ILs where the length of the alkyl chain R was varied. The ILs were prepared using azide-alkyne ‘click’ chemistry. The 1,2,3-triazolium ILs that resulted from our efforts exhibited excellent thermal stability properties (> 325 °C) with relatively low viscosities. What was most surprising was that the 1,2,3-triazolium ILs were found to exhibit ionic conductivities which were comparable to the analogous imidazolium systems and were far superior to the 1,2,4-triazolium ILs (Figure 2). Such a trend can be directly related to the relative Lewis acidities of the cationic rings. The results of this study were published in Tetrahedron Letters (January 2016) and have further motivated us to pursue the 1,2,3-triazolium cation in future IL and PIL studies.
Project II: Poly(ionic Liquid)s for Electroactive Applications (full ACS-PRF support):
Poly(ionic liquid)s (PILs) represent an intriguing class of solid polymer electrolytes in that they combine the unique thermal, chemical and conductive properties of ionic liquids with the mechanical stability of various macromolecular designs. PILs have received a significant amount of attention in a variety of energy-oriented applications, including fuel cells, batteries and carbon dioxide separations.
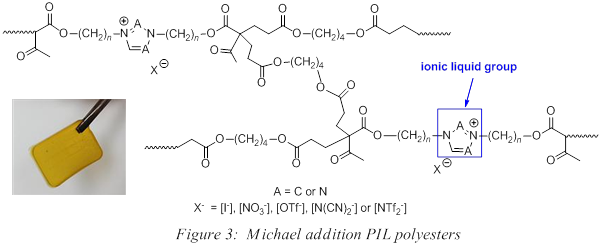
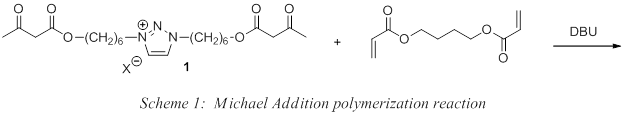
Our contributions to the area of PILs have focused on the generation of polyester networks with the general structure in Figure 3. To prepare these systems, we employ a Michael addition polymerization strategy where an ion-containing, multi-functional acetoacetate reacts with a multi-functional acrylate under basic conditions. Over 50 covalently crosslinked PIL networks containing an imidazolium or triazolium group have been successfully prepared using this strategy. Recent efforts have focused on the incorporation of the 1,2,3-triazolium group into these Michael addition PIL esters, leading to the synthesis of monomers 1 which were subsequently polymerized with commercially available 1,4-butanediol in the presence of catalytic base (DBU) (Scheme 1). The resulting PILs appeared as transparent, elastomeric materials with varying degrees of yellow.
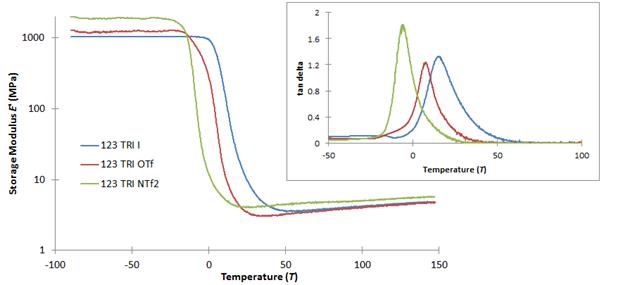
Thermal and mechanical properties of all of the 1,2,3-triazolium PIL polyesters were determined using differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and dynamic mechanical analysis (DMA). Correlations between monomer ratio/crosslink density, cation type (imidazolium vs. triazolium), alkyl spacer length and counteranion (iodide [I-], triflate [OTf] and [NTf2]) with properties such as glass transition temperature (Tg), thermal stability, crosslink density and storage modulus (E′) have been made, the results of which are currently being summarized in manuscript form. We found that using a bulky, non-coordinating anion such as [NTf2] led to the highest thermal stabilities (> 240 °C). As expected, Tg values were highly dependent upon the size of the anion. DMA measurements indicated that all PILs prepared exhibited similar crosslink densities with storage modulus (E′) values of 4-5 MPa at 100 °C and a high degree of uniformity from the shapes of the tan delta curves (Figure 4)
Figure 4: DMA comparison of selected 1,2,3-triazolium-containing PIL polyesters
Future Work:
Although our UNI grant ended this year, the next six months will be spent finishing the acquisition of thermal and mechanical data for the purposes of publication. Additionally, we will begin to explore the ionic conductivities of these (and other) Michael addition PILs using dielectric relaxation spectroscopy in order to provide information about ion transport and polymer dynamics. Furthermore, we have also started an internal collaboration in order to explore the utility of our PILs as carbon dioxide separation membranes.