Reports: UNI354247-UNI3: Non-Precious Metal Catalytic Asymmetric Reduction of Unsaturated Bonds: A Supramolecular Approach
Guoqi Zhang, PhD, John Jay College of the City University of New York
Overview of Research Project. A current challenge in the field of renewable and sustainable chemistry is the replacement of precious metal catalysts with earth-abundant metal alternatives. With precious metals (Pd, Pt, Ru, Rh and Ir) comprising nearly all of the most effective catalysts, new abundant alternatives are needed to promote useful catalytic processes including hydrogenation, hydrofunctionalization and cross-coupling reactions pertinent to large-scale chemical transformations. This project supported by the PRF grant during 2014-2016 has allowed the PI to make significant contributions to the development of new catalyst systems based upon abundant, nontoxic metals (cobalt and manganese) to promote several important chemical transformations involving hydrogenation and dehydrogenation processes. The UNI grant also supports sufficient involvement of undergraduates, in particular women and those from underrepresented groups to participate in cutting-edge research in this fast growing topic in the PI’s laboratory. Preliminary results have been obtained in the funding period, leading to nineteen journal publications and two full grant applications submitted to federal agencies.
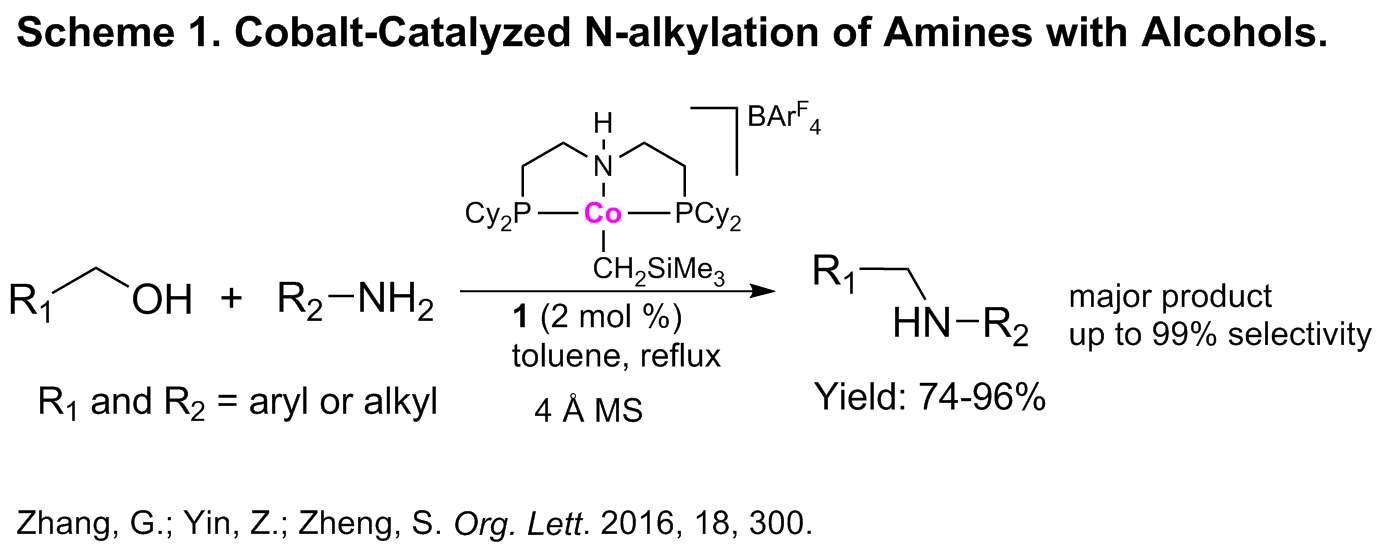
Current Progress. The proposed work was conceived based upon the PI’s previous observation on a highly active CoII(PNP)(alkyl) complex that enables the hydrogenation and transfer hydrogenation of C=C, C=O and C=N bonds of a number of substrates under mild conditions. Supported by the PRF grant, we have been able to extend the research significantly. The catalytic N-alkylation of amines with alcohols offers a green and atom-economic pathway for the synthesis of substituted amines that is critical in organic and medicinal synthesis. Previously, precious metal Ir- and Ru-catalyzed N-alkylation of amines by alcohols has been reported by several groups and it was suggested that a “hydrogen-borrowing” mechanism is responsible for the successive dehydrogenation/hydrogenation process. Our work revealed that cobalt(II) catalyst (Scheme 1) was effective for the direct N-alkylation of both aromatic and aliphatic amines with alcohols. It was shown that a subtle change in reaction conditions (adding 4 Å molecular sieves to the reaction system) was the key to a switch in the resulting products (amines vs. imines) with high chemoselectivity of amine products. A range of alcohols and amines including both aromatic and aliphatic substrates were converted to secondary amines in good-to-excellent yields when 2 mol% cobalt catalyst was used. Remarkably, no base additive was required for the high efficacy. Additional experiments also indicated that a hydrogen-borrowing mechanism was responsible for the tandem acceptorless dehydrogenation/imine formation/hydrogenation process. This work represents an importance advance in cobalt-catalyzed C-N bond formation via a green catalytic process.
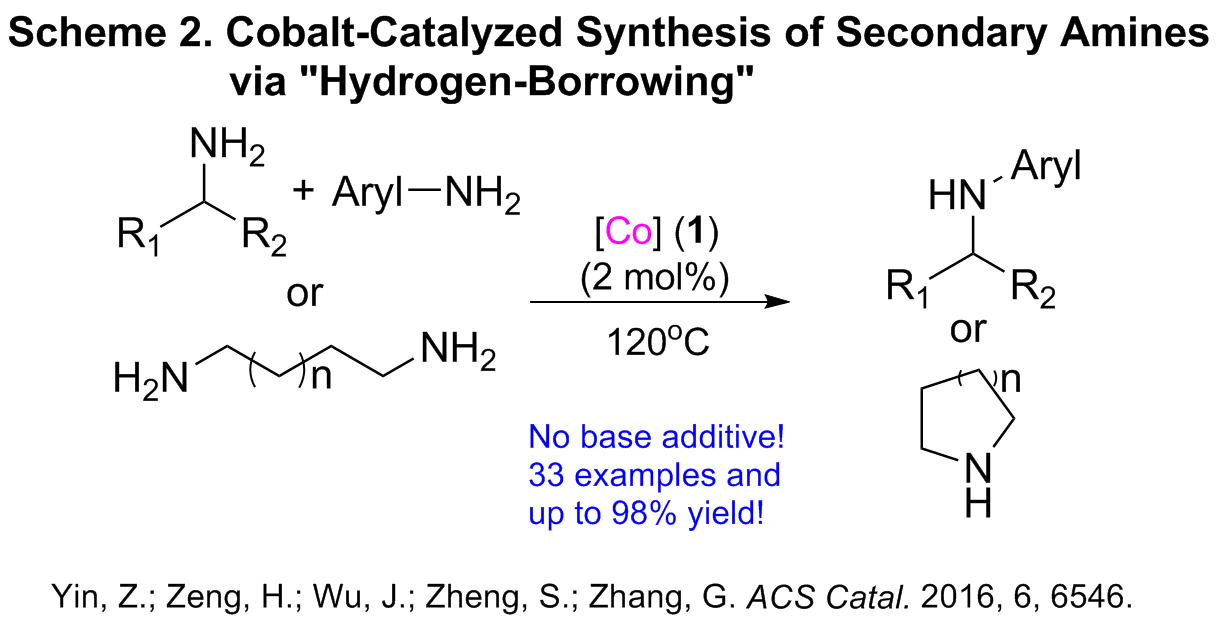
Following this work, we also investigated the possibility of the alkylation of amines by amines by using the same catalyst. Although the selective mono-alkylation of amines with other amines has been only carried out previously by using precious metal catalysts, our recent work reveals that cobalt pincer complex is highly active for the catalytic alkylation of amines with other amines through a “hydrogen-borrowing” methodology for the synthesis of a broad range of aromatic, aliphatic secondary amines, and cyclic sec-amines were also achieved as rare examples (Scheme 2). The well-defined cobalt pincer complex (2 mol% loading) was observed to show comparable catalytic activity and efficiency to the known Ru and Ir catalysts for similar transformations, even at lower reaction temperature. This cobalt-catalyzed intramolecular alkylation of linear diamines is significant as it affords cyclic amines that are pharmaceutically important intermediates.
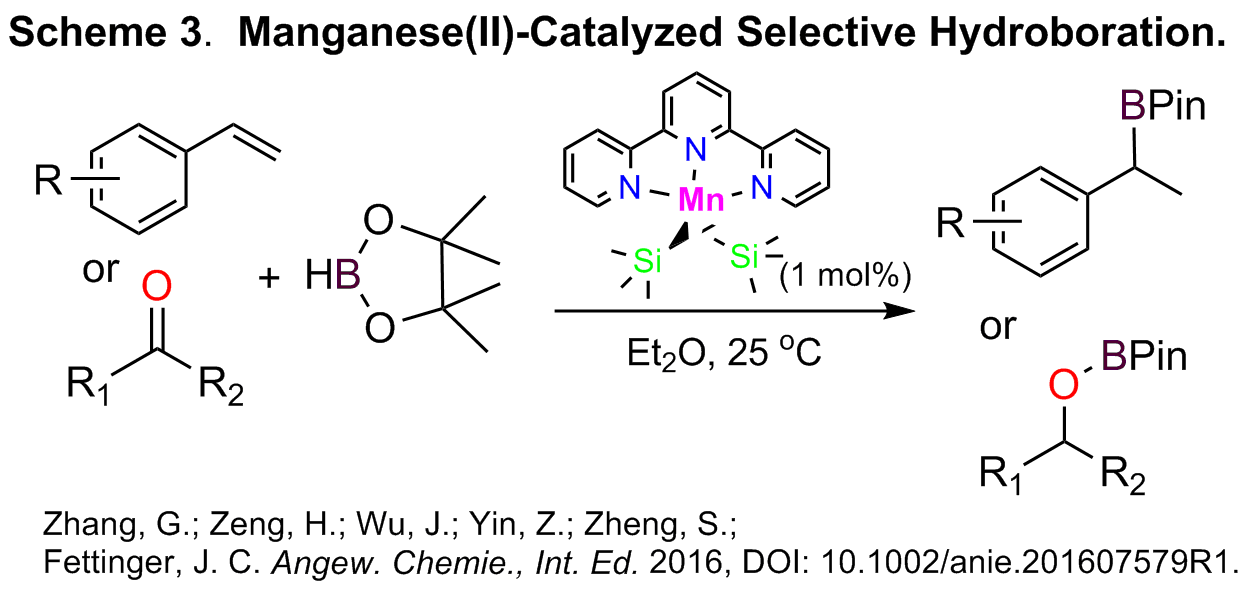
Under the support of this award, we continued to work on the development of new metal catalysts based on earth-abundant metals and have recently explored the possibility of utilization of another important and highly abundant metal, manganese. Next to iron and titanium, manganese ranked the third most abundant transition metals in the Earth’s crust. Surprisingly, it was almost unknown for catalytic reduction reactions until several recent reports in 2016, although it has long been known as efficient catalysts in oxidation process. In addition, the synthesis of boronate esters through the hydroboration of unsaturated bonds is an important research area. Metal-catalyzed hydroboration of alkenes has proven to be an atom-economical and selective route to valuable alkylboronate esters, and therefore, the development of metal catalysts for this conversion in a regio- and/or stereo-selective manner has attracted considerable attention. However, examples involving base metal catalysts for Markovnikov selective hydroboration of alkenes are extremely rare. Remarkably, our work reveals for the first time a well-defined manganese-catalyzed hydroboration of alkenes, ketones and aldehydes (Scheme 3). The manganese catalyst we described could be readily prepared from an inexpensive, readily available ligand, 2,2′:6′,2′′-terpyridine (NNN instead of PNP), and the catalytic hydroboration is highly efficient under mild conditions, giving unusual Markovnikov selectivity for a range of styrene derivatives. In addition, excellent chemoselectivity of the manganese catalyst towards ketones over alkenes was achieved. This work represents a significant progress on manganese catalysis. We are currently investigating the experimental and computational aspects that will lead to a mechanistic elucidation regarding the selective hydroboration, and also developing enantioselective hydroboration catalysts based on Mn or Co.
Impact. The utilization of nonprecious metals in catalysis makes our synthesis more sustainable and advantageous over those reported. The current project on non-precious metal catalysis provides arrays of opportunities for undergraduate researchers to conduct cutting-edge research at John Jay College, a certified Minority- and Hispanic-Serving Institution and a Primarily Undergraduate Institution recognized by National Science Foundation. The existing science Program for Research Initiatives for Science Majors (PRISM) within the college partially benefits this project by providing additional financial support to our undergraduate researchers. Five undergraduates (4 females, 1 male) have worked on relevant projects supported by this award and they have coauthored in 5 recent publications, which help the students to pursue a chemistry PhD degree after graduation. Posters from their work have been presented at both university research symposium and nation-wide conferences. As a new faculty at a primarily undergraduate institution the PI has greatly benefited from this award through transitioning to the next stage of his career, while pursuing tenure and promotion.