Reports: DNI1055036-DNI10: Fundamental Investigation of Inorganic Metal Borophosphides
Kirill Kovnir, PhD, University of California, Davis
In the course of this project the students involved learned the basic solid state synthetic techniques and their limitations. The necessity of the in-situ characterization for the development of the advanced synthetic methods is crucial. We have chosen an in-situ X-ray powder diffraction method. To learn this technique we attended several beam lines at the Argonne National Lab Advanced Photon Source 17-BM beamline. In the course of this project we have resolved a number of the technical issues regarding samples handling, environment, etc. Those investigations have a significant impact on our global view of the synthetic landscape and allow for non-standard approaches towards novel compounds.
In our exploration of new compounds containing a B-P bond, we have discovered alternative low temperature synthetic routes to cubic boron phosphide. These methods are an improvement to previously reported methods that require high temperatures of over 1000°C and up to three weeks of annealing.
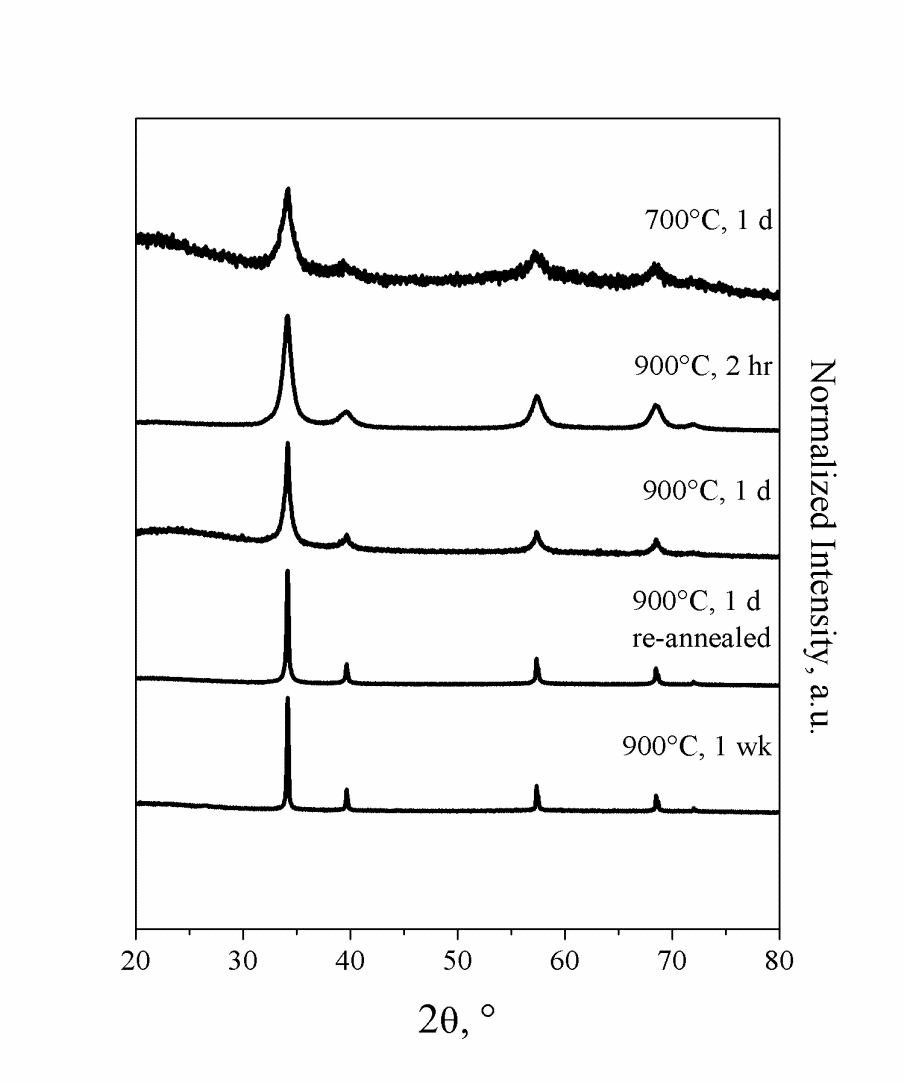
Phase pure polycrystalline cubic BP has been synthesized by metathesis of BI3 and P in an evacuated and sealed silica ampoule at 900°C for ~20 hours. This reaction generated BP with P2I4 as a side product that could be washed away with water. Fibrous P21 was also found to be a side product, though it could be segregated from the product by applying a temperature gradient of ~60°C to the reaction ampoule. The colder end of the ampoule containing P21 could then be separated from the desired product and discarded. The resulting BP was re-annealed at 1050°C for three days to further improve crystallinity. We have found that BP formed at a temperature as low as 700°C with only a day of annealing, although the product had poor crystallinity. Increasing temperature and length of annealing only improved the crystallinity of the product (Figure 1).
Figure 1. Powder X-ray diffraction (XRD) patterns of BP synthesized by solid state metathesis varying temperature and annealing time.
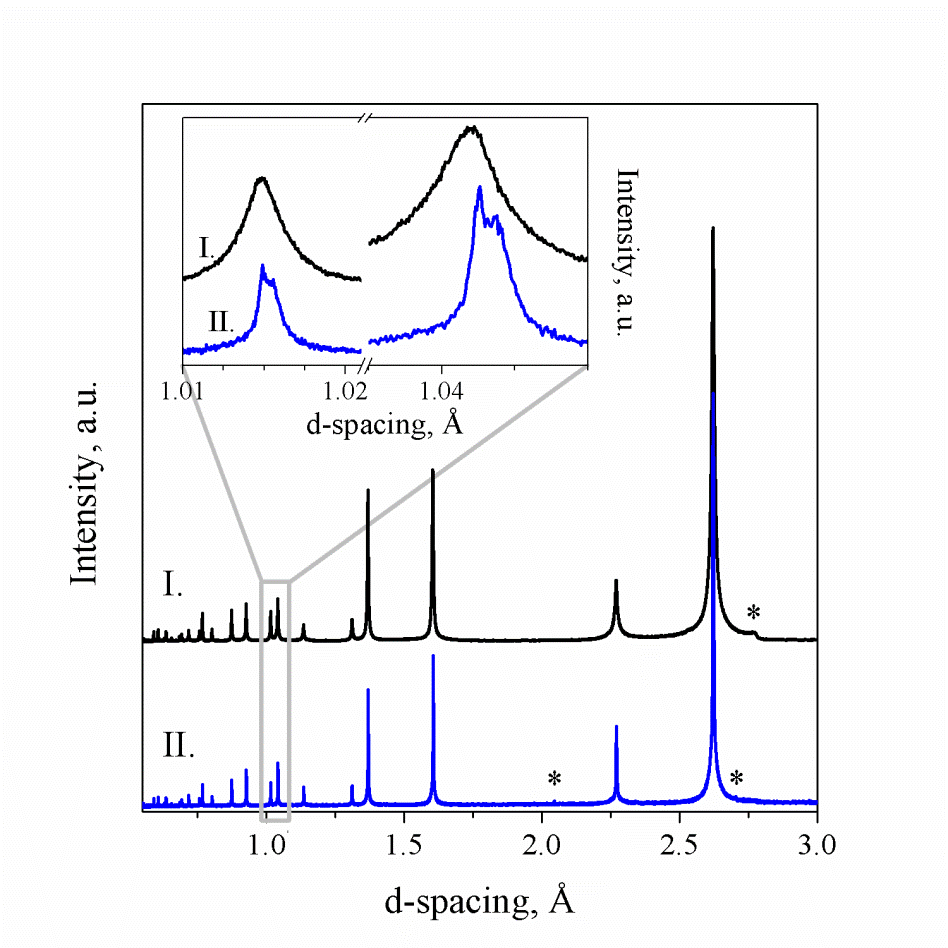
A ternary compound, Sn-doped BP was synthesized by flux method starting from amorphous boron and red phosphorus and tin excess in an evacuated and flame sealed silica ampoule. After the reaction, Sn flux was removed by high temperature centrifugation, followed by washing in a 1:1 HCl:H2O bath. Unit cell parameters determination together with energy dispersive X-ray spectroscopy revealed that ~2% Sn incorporates into the BP structure, which was further confirmed by the synchrotron X-ray diffraction at the APS beamline 11-BM (Figure 2). An analysis of the products of oxidation of Sn-doped BP indicates that tin is located in boron sites, forming SnxB1-xP. High resolution XRD revealed that two different compounds with distinct unit cells have been formed in the Sn flux. In addition, any crystalline boron from the starting material tended to remain unreacted (Figure 2). Although Sn incorporation is undesirable for the synthesis of single phase binary BP, this doped compound can be recognized as a new metal borophosphide, an uncommon category that contains less than 10 inorganic compounds with B-P bonds.
Figure 2. Powder synchrotron XRD patterns of I) BP produced by high-temperature reaction of elements and II) BP synthesized in tin flux. Inset shows enlarged fragments of both patterns. Asterisks indicate diffraction peaks of the crystalline boron.
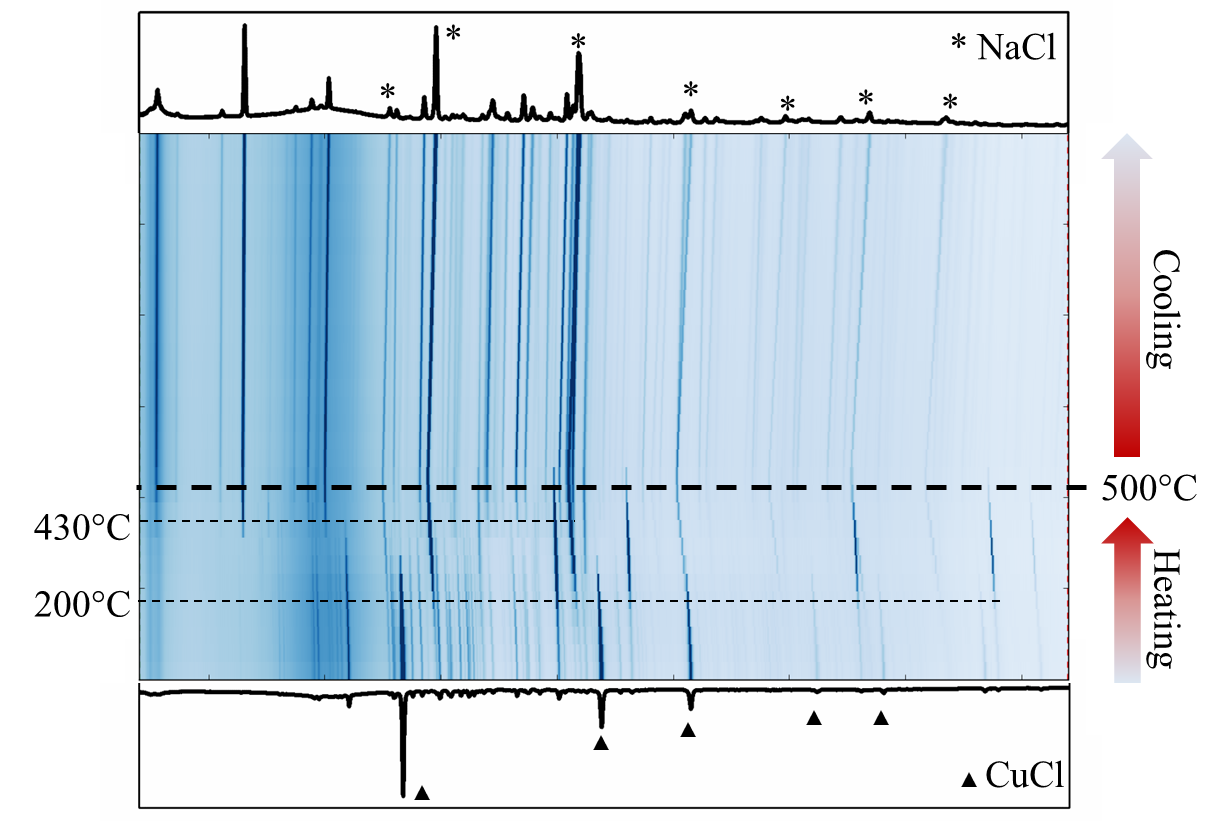
Because of the uncommon nature of metal borophosphides as well as their potential technological properties we are investigating the synthesis of new metal borophosphides. Previous attempts to produce these new compounds by traditional solid state methods such as high-temperature reaction of elements or flux synthesis were unsuccessful and only resulted in binary phosphides and borides. As such, we explored reactions by solid state metathesis, which were proven to be successful in synthesizing new metal carbodiimide compounds. Na3BP2 was synthesized as a precursor containing P-B-P fragment with covalent B-P bonds. This precursor was then reacted with various transition metal halides based on the hypothesis that the B-P bond would be preserved while the metal ion would exchange. The common result when using first row transition metal chlorides was the formation of NaCl, indicating the exchange reaction took place, as well as various metal phosphides and an unidentified compound as given by powder X-ray diffraction. When washed with water, binary BP was detected instead of the unknown compound, suggesting the presence of a B-P bond in the unknown compound. We chose to focus on the reaction with CuCl. Through the recent in-situ powder synchrotron X-ray diffraction experiments at the Advanced Photon Source beamline 17-BM, we found that the starting materials react already at temperatures as low as ~200°C, while the target ternary of quaternary Cu-B-P compound began to form at ~430°C and decomposes at ~600°C. Using the collected temperature stability data, we are devising a synthetic experiment for growing single crystals of new phase so that the crystal structure and composition can be properly determined.
Figure 3. In-situ powder XRD of Na3BP2 reacting with CuCl.