Reports: UR155707-UR1: Organocatalytic Domino Reactions for the Synthesis of Highly Substituted Heterocycles
Shanina Sanders Johnson, PhD, Spelman College
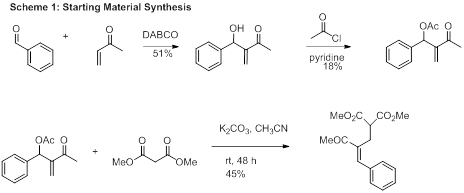
During the 2015-2016 academic year three students contributed to the research goals of this grant. The initial focus for this project included making the required Morita-Baylis-Hillman (MBH) adduct and exploring its reactivity with a variety of electrophiles. Although the steps for this starting material were outlined in the literature, performing these procedures has not resulted in the reported yields. Therefore, the students have spent some time optimizing the starting material synthesis. Currently the MBH reaction is being performed with polyethylene glycol as a solvent. The reported procedure gave an 89% yield for this transformation. A 51% yield has been achieved in our lab with considerable benzaldehyde starting material remaining. MBH reactions are notorious for their low yields and long reaction times. In this case the methyl vinyl ketone may be involved in a side reaction that forms a by-product. This by-product is difficult to remove via column chromatography and is carried on to the acetylation after purification. The acetylation is performed using acetyl chloride as acetic anhydride does not give the desired product with our substrate. Low yields around 20% plague this reaction. The final substitution step takes longer than the 24 hours reported. After stirring for 48 hours the product can be obtained in about 50% yield after purification. Starting material can be recovered cleanly from this reaction but increasing the reaction time has not resulted in higher yields thus far. The three-step sequence is outlined in Scheme 1.
Synthesis of Piperidines
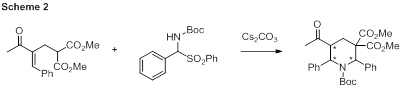
Piperidines were one of the targets we have attempted to synthesize. With the MBH adduct in hand, α-amido sulfones were chosen as a reaction partner to form piperidines. α-amido sulfones are ideal for undergraduate synthesis because they are stable and easy to handle. They generate an imine in situ, so their reactivity is not hindered. One undergraduate was able to synthesize over eleven grams of the α-amido sulfone shown in Scheme 2. This material is currently being investigated for reactivity with the MBH adduct. Thus far, there seems to be reactivity in methylene chloride and toluene but a thorough analysis of the results is ongoing as the reaction is quite messy by 1H NMR analysis. Some starting material can be seen but a variety of new peaks are formed. The reaction mixture has not been purified so the individual components of the mixture have not been identified, however a short solvent screen is underway to see if a cleaner reaction with full conversion of the starting material occurs in the presence of a different solvent.
Synthesis of Tetrahydropyrans
The synthesis of tetrahydropyrans was also attempted. Electrophiles that have been utilized with the MBH adduct are benzaldehyde, ethyl glyoxylate, and indole. A short screen of bases including potassium and sodium carbonate did not yield any reactivity with any of the electrophiles. These bases are known to be somewhat insoluble so different solvents were also attempted to promote solubility. Thus far, no reactivity has been detected with these systems. The starting materials remain intact by 1H NMR analysis. In an effort to promote reactivity, a stronger base, sodium hydride, was used. Some starting material was still present after the work up under these conditions as well. The next steps for this transformation include using a more active electrophile such as the ethyl glyoxylate with the stronger base. The equivalents of base will also be varied to promote nucleophilic attack. Finally, DBU will be used as a base since it has shown to be active with this type of MBH adduct with electron deficient alkenes. Two equivalents of DBU were needed and other bases such as K2CO3 and Cs2CO3 were not reactive as is the case in our system as well.1 In addition to continuing work on the previously mentioned projects, the reactivity of the MBH adduct will be further probed by heating the reactions. A microwave synthesizer will be employed to afford quick reaction times which are very desirable for the undergraduate schedule.
Student Training
As part of the research experience students have been introduced to advanced literature searching and databases such as SciFinder. We have group meetings where we have discussed scientific ethics and professional norms of the field. In addition, the students were advised on career paths, resumes, and other academic goals. Two of the students presented as Spelman’s annual Research Day in April of 2016 and the third submitted a formal report via her senior research course. Two of those students have since graduated. They often discussed their concerns or asked questions about graduate school and professional school applications over the course of the year. One is attending graduate school at the University of Arkansas Fayetteville in the Chemistry and Biochemistry Department. The other was accepted into a post-baccalaureate program at the University of Michigan but did not attend due to financial concerns.