Reports: UNI955668-UNI9: Kinetic Studies to Optimize Chemical Dissolution and Inhibition of Common and Exotic Oilfield Scales
Jessica M. Wilson, PhD, Manhattan College
Project Objective and Background
The objective of this research is to optimize the conditions for using chelating agents/phosphonate based chemicals to dissolve and inhibit common and exotic oilfield scales (SrSO4, ZnS and PbS). Inhibition and dissolution of exotic mineral scales, such as SrSO4, PbS, and ZnS are minimally studied. Thus, chemical dissolution and inhibition of these scales, which significantly decrease well production and damage equipment, can ensure continued oil well production.
Work Conducted
Task 1: Dissolution of SrSO4, PbS, ZnS
Dissolution experiments of SrSO4 using the chelating agent EDTA were stirred batch tests. SrSO4 was crushed and dried and was added to an EDTA buffered solution. Time series samples were analyzed for the presence of the Sr-EDTA complex (confirmation of metal-chelate complex formation), free Sr2+, and free SO42. Sr-EDTA was determined analytically using capillary electrophoresis, free Sr2+ using flame atomic absorption spectroscopy and free SO42- using ion chromatography.
The experimental matrix is shown in Table 1. The experiment was conducted for 3 hours (in duplicate) with samples collected at times 0, 0.25, 0.5, 1, 2, and 3 hours.
Table 1. Experimental conditions for SrSO4 dissolution. 5 mM KCl used to buffer solution to desired pH.
Experiment | Mass of SrSO4 (g) | [EDTA] (mM) | pH |
1 | 0.5 | 5 | 12 |
2 | 0.5 | 5 | 9 |
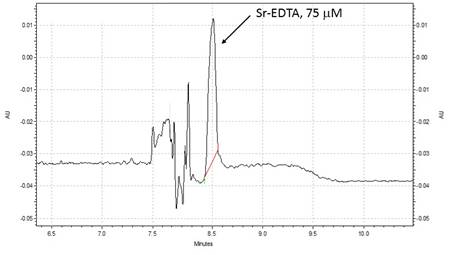
The presence of the Sr-EDTA complex is shown in the sample electropherogram at time 2 hours for Experiment 01 (Figure 01). This complex was confirmed by comparing electropherogram with prepared Sr-EDTA standards.
Figure 1. Representative electropherogram showing Sr-EDTA formation during Experiment 1. Background electrolyte (BGE): 20 mM sodium borate, 1 mM EDTA, 5% ethylene glycol; Voltage 12.5 kV. UV detection at 214 nm.
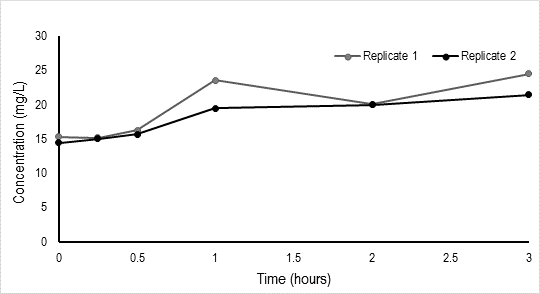
Additionally, the concentration of free Sr2+ increases over 3 hour period for the two replicate batch tests in Experiment 1 indicating that the SrSO4(s) complex is dissolving (Figure 2).
Figure 2. Concentration (mg/L) of Sr2+ versus time (hours).
A preliminary chemical reaction kinetics model following Dunn et al. (1999) will be followed to determine reaction rates for the dissolution of SrSO4(s) after additional data has been gathered.
The next steps in this work are to optimize the dissolution of SrSO4(s) by 1) assessing the effect of temperature on the dissolution rates of SrSO4(s); 2) assessing the effect of an alternate ligand (DTPA) and different ligand concentrations on SrSO4(s) dissolution; 3) optimizing the dissolution of the exotic scales PbS(s) and Zn(s) following methods used to dissolve SrSO4.
Task 2: Inhibition of SrSO4, PbS, ZnS
Significant progress has been made in the precipitation of SrSO4, X-ray diffraction (XRD) analysis of SrSO4 and MINEQL+ modeling to determine the optimum effects of chelating agents. After optimization of SrSO4 inhibition, inhibition of PbS and ZnS will be conducted in Year 2.
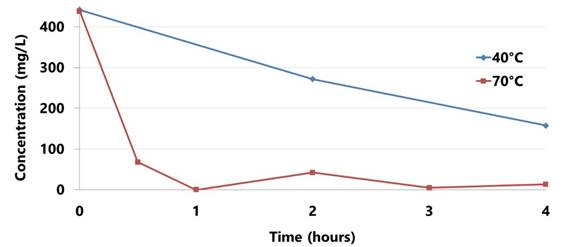
Precipitation of SrSO4 in presence of Sr2+ and SO42- has been optimized. Bench scale precipitation experiments have been conducted in closed Teflon flasks with a magnetic stirrer at constant stirring rate. It showed that the rate of Sr2+ removal is significantly higher at higher temperature (70°C) compared to lower temperature (40°C) over 4 hrs period. 420 mg/L of Sr2+ was removed completely within 1 hr period at 70°C compared to minimum removal at 40°C (50% Sr2+ removal within 4 hrs period). A white precipitate (SrSO4) formed under both temperature conditions (40°C and 70°C).
Figure 3. Concentration (mg/L) of Sr2+ versus time (hours) from precipitation of SrSO4.
Chemical speciation modeling, using MINEQL+, is being employed. Saturation index has been used as indicator of supersaturation or under-saturation of SrSO4. The following formula has been used for Saturation index calculation:
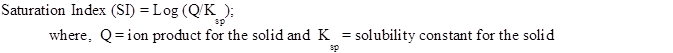 |
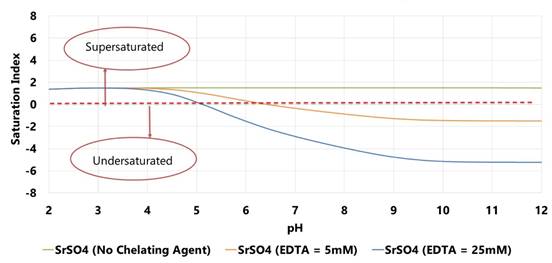
MINEQL+ modeling experiment has been conducted for 5 mM Sr2+, 5 mM SO42-, 100 mM NaCl in presence of EDTA. Two concentrations of EDTA have been modeled: 5 mM and 25 mM. Based on the MINEQL+ results, SrSO4 is under-saturated at high pH (11-12) in the presence of chelating agent (EDTA). Saturation indices of SrSO4 decreases as the concentration of chelating agent (EDTA) increases.
Figure 4. Saturation indices for SrSO4 at 5 mM Sr2+, 5 mM SO42-, 100 mM NaCl in the presence of EDTA.
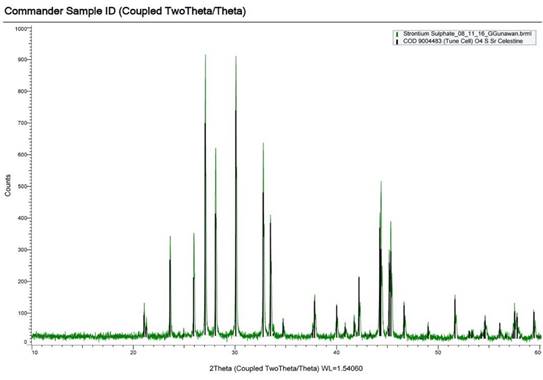
Significant effort has been utilized to train the students to get students familiar with XRD analysis. Currently, the precipitate characterization of the oilfield scales is being confirmed using XRD (X-ray diffraction) techniques. Commercial SrSO4 sample has been analyzed already using XRD shown in Figure 5.
Figure 5. XRD comparison of commercial SrSO4 salt with database peaks of SrSO4.
The next steps in this work are further modification and optimization of inhibition of oilfield scales with phosphonate-based chemicals. Furthermore, precipitation of ZnS and PbS to be conducted in the laboratory for bench scale experiments of inhibition and dissolution. Effort is underway to collect and characterize the produced water and its subsequent microcosm studies with different scales and optimized chelants/inhibitor.
Broader Impacts
This research has had significant impacts for the PI and co-PI as well as their research students. The PIs acknowledge that without this funding they would not be able to pursue research in this area, and have explored options for additional funding once this project is complete. This will have a significant impact on their careers as they have expanded their research scopes. The PIs have supported three undergraduate students and one graduate student through this funding. Two of the students recently presented their results at an on-campus conference for undergraduate student research. It is anticipated that these students will also present this work at a regional or national conference and contribute to this work as it nears publication in a peer-reviewed journal. Contributing to this research has sparked one student’s interest in pursuing graduate work and will greatly benefit both students as they pursue graduate work/careers in environmental engineering and chemistry after graduation.