Reports: UNI654592-UNI6: A Physicochemical Exploration of the Diffusion of Small Molecules in Glassy and Highly-Viscous Materials
Andrew J. Huisman, PhD, Union College
During the period September 2015-August 2016 I was able to finish rebuilding the research apparatus and brought it online in early summer 2016. As of this writing, the instrument is able to perform precision sizing measurements. During the reporting period, I worked with three students (two chemists and an engineer) on this project.
The two chemistry students worked around 4-6 hours per week during the academic term, helping with instrument construction, troubleshooting, and alignment. They worked separately to allow me to spend time with each of them, one-on-one. Their role was mostly to help test the instrument by e.g., optimizing the spectrometer slit width to get the best signal to noise, helping to write and test instrument control code, and helping with alignment of optics and performing injections of particles.
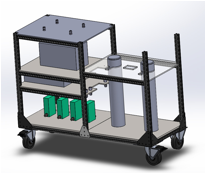
The engineer worked full time during the summer term (8 weeks) and was tasked with reimagining the gas delivery system for the electrodynamic balance (EDB) so that it could provide vapor saturated with water or with short-chain alcohols such as methanol or ethanol. The purpose of this redesign was to allow me to measure diffusivity of a homologous series of small molecules (methanol, ethanol, propanol) with the aim of exploring the strengths and weaknesses of using small molecules as tracers for diffusion. I met with the engineer almost daily but allowed him to have freedom to work on the project. We decided on the design criteria together and he then worked independently to propose solutions that would produce reliable, reproducible, aerosol-free vapor streams. The final design is a bubbler system, as shown in Figure 1.
Figure 1: Design produced by undergraduate engineer for a dual-bubbler system to safely, reliably, and reproducibly introduce alcohol vapors into a gas stream.
In order to make high-quality measurements of diffusivity in droplets, three systems are needed. First, a means of introducing water and alcohol vapors to the gas-phase in the apparatus (the bubbler system above); second, high-precision sizing via Mie Resonance Spectroscopy; second, third, a means of quantifying the actual concentration of vapors in the cell. The first system has been designed and is under construction. The second system is working, as detailed below. The third system will be realized using a chilled-mirror hygrometer that has been ordered and is expected to arrive by mid-October 2016.
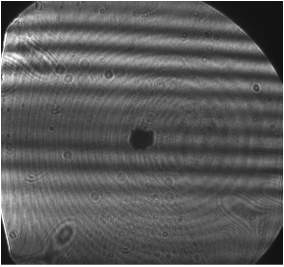
Using Mie resonance theory in the geometric optics limit, it can be shown that the scattering pattern for a particle in the size range 1-10 microns from laser light (of a very narrow range of wavelengths) will form a series of light and dark bands with a spacing that is inversely proportional to the radius of the particle as shown in Figure 2.
Figure 2: Fringe pattern showing horizontal light-dark pattern. Larger particles have a more tightly spaced pattern.
While it is in theory possible to directly calculate the size of the particle using geometric optics and the spacing of the scattering pattern shown in Figure 2, it is in practice preferable to inject particles of a known size and measure their spacing. Latex sphere of various sizes between 1.1 and 9.6 um have been purchased and will be used to calibrate the sizing measurement.
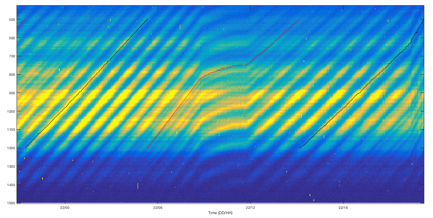
The fringe pattern shown in Figure 2 allows us to directly determine the absolute radius with moderate precision (±20%) but high-precision sizing is needed to make measurements. This is accomplished by observing the wavelength-dependent scattering as a function of time and tracking the movement of resonances. The data shown in Figure 3 demonstrate that the system is acquiring spectra and tracking them successfully. This system is not kinetically hindered as the LED spectra move in concert with the change in humidity; in systems that have limited diffusivity, the LED spectra will lag behind the change in humidity.
Figure 3: False-color LED spectra overlaid with tracking (dotted line) showing the relative size of a particle changing over time. The particle has a moderate vapor pressure and is evaporating (indicated by the upward trend). In the center of the figure, the humidity was very slowly increased, partially offsetting the evaporation and leading to a smaller rate of shrinking.
I hope that by the beginning of the 2017 calendar year it will be possible to observe diffusionally-limited growth of droplets by making large changes in humidity quickly and using droplet materials with a higher glass transition temperature such as sucrose or levoglucosan.
The support of the ACS PRF has allowed me to mentor three undergraduate students and will lead to at least one presentation at our internal Steinmetz Undergraduate Research symposium.