Reports: DNI555170-DNI5: Inorganic Capping Synthesis of Pure Phase Intermetallic Nanoparticles for Non-Oxidative Propane Dehydrogenation
Wenyu Huang, PhD, Iowa State University
Research in the Huang Group focuses upon the design and synthesis of well-defined nanostructures for studies and applications in heterogeneous catalysis. Using well-defined nanocatalysts, our long term goal is to understand the structure-catalytic property relationships, which will be used to guide the future design of catalysts to achieve efficient, economical, and environmentally friendly chemical transformations.
Impact of Proposed Research. Precious metals and metal alloys are important heterogeneous petrochemical catalysts. However, they have their limitations. Precious metals have low natural abundance. Metal alloys have unstable surfaces due to surface segregation under reaction conditions, which renders the identification of active sites and an understanding of reaction mechanisms difficult. This research project is designed to address these limitations by using pure phase intermetallic nanoparticles (iNPs). The inherent properties of intermetallic compounds, often thermally stable and exhibiting a large variety of structures, will help us to clarify many proposed mechanisms that the formation of IMCs is responsible for the enhanced catalytic properties in many heterogeneous catalysts.
Intermetallic compounds (IMCs), consisting of two or more metallic/metalloid elements, adopt specific, well-defined crystal structures that are distinctly different from random alloys, and have different electronic structures in comparison to their constituent elements. The modified electronic structures of IMCs make them enticing catalytic materials because they will alter the binding energies of surface adsorbates (reactants, intermediates, and products). Using IMCs as heterogeneous catalysts could lead to improved catalytic properties as well as the reduction or even replacement of catalysts based on precious metals. Considering the availability of IMCs with different structures (~100,000 discovered so far), their catalytic properties remain largely unexplored.
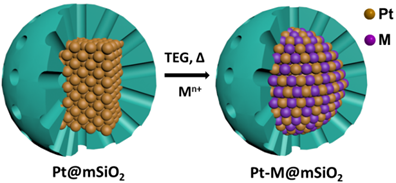
Figure 1. Converting mSiO2-encapsulated Pt NPs to PtM iNPs.
Our efforts started with the first-time discovery that NaAu2 IMC showed room temperature catalytic activity for carbon monoxide (CO) oxidation (J. Am. Chem. Soc. 2013, 135, 9592-9595.). Density functional theory (DFT) calculations suggested that the low-temperature activity could be due to enhanced O2 adsorption on the NaAu2 surface. However, the NaAu2 sample, prepared using a traditional high temperature method, gave bulk material with a very low surface area (< 0.1m2/g) that is not preferred for catalysis. To prepare iNPs with high surface-to-volume ratios, we developed a new method to make iNPs using a “ship-in-a-bottle” strategy. (Nanoscale 2015, 7, 16721-16728.)
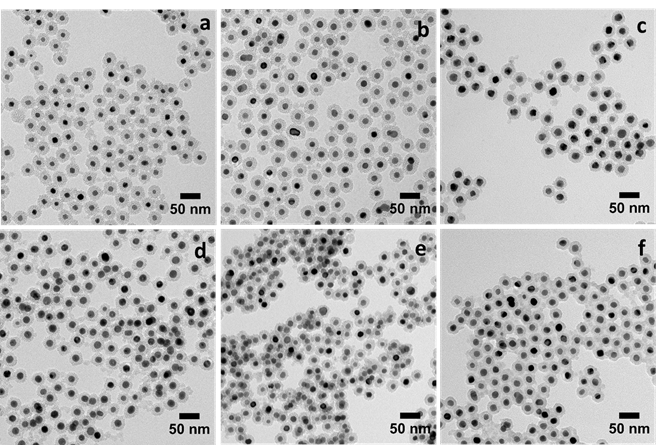
In traditional colloidal synthetic methods, organic capping agents are usually needed to prevent the aggregation of nanoparticles (NPs). Because most organic capping agents only provide limited protection against aggregation of the NPs at the high temperature (~300°C), which is necessary for the formation of pure phase iNPs, we proposed to use inorganic capping shells to protect pure metal NPs and convert them to iNPs at the high temperature required for the incorporation of the second metal to form IMC phases (Figure 1). The inorganic capping shells have a porous structure, which does not prevent diffusion of the second metal to the core. For the first time, we have successfully synthesized Pt3Sn, PtSn, PtPb, Pt3Zn, and PtZn iNPs encapsulated in mesoporous silica (mSiO2) using this ship-in-a-bottle strategy (Figure 2). These mSiO2 shell protected iNPs do not aggregated below 750°C (ACS Catal. 2016, 6, 1754-1763.).
Figure 2. TEM images of (a) Pt@mSiO2 and (b-f) intermetallic PtxM@mSiO2 NPs for (b) Pt3Sn; (c) PtSn; (d) PtPb; (e) Pt3Zn; and (f) PtZn.
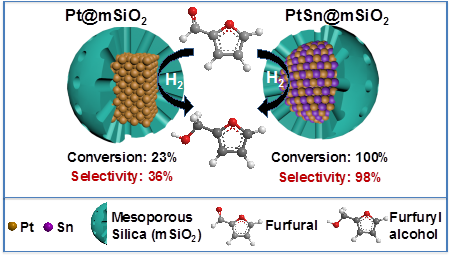
We chose selective hydrogenation of furfural as the model reaction to evaluate the catalytic properties of these iNPs. The structure of furfural molecule (C4H3OCHO) is shown in Figure 3. Furfural can be converted to either furfuryl alcohol through hydrogenation or furan/methyl furan through hydrogenolysis, and is, thus, an ideal molecule for studying hydrogenation/hydrogenolysis selectivity. The intermetallic PtSn@mSiO2 (with a 1:1 molar ratio of Pt:Sn) exhibited superior activity and selectivity for hydrogenation of furfural to furfuryl alcohol in comparison to Pt@mSiO2 (Figure 3). The specific activity per surface Pt site on PtSn@mSiO2 is about 40 times that of Pt@mSiO2. In IMCs Pt benefits from an ordered environment, which is deemed responsible for the observed high activity and selectivity in the hydrogenation of furfural to furfuryl alcohol. Our work has demonstrated that greatly enhanced activity and selectivity can be achieved using iNP catalysts while reducing precious metal usage, and silica encapsulation can effectively prevent aggregation of PtSn NPs during catalyst synthesis and regeneration.
Figure 3. Comparison of the conversion and selectivity of mSiO2-encapsulated Pt and PtSn in furfural hydrogenation. Using Pt as the catalyst, only 36% of the converted furfural goes to furfural alcohol and the rests are hydrogenolysis products. In contrast, we can achieve quantitative conversion of furfural to furfural alcohol using the intermetallic PtSn@mSiO2.
This “ship-in-a-bottle” strategy provides a new route to synthesize iNP catalysts with a broad spectrum of compositions and uses. Currently, we are making Pd-, Rh-, and Au-based iNPs using this strategy for selective oxidation and reduction reactions. Research in this area will allow us to establish the correlation of catalytic properties of iNPs with their surface geometric and electronic structures. This knowledge will serve as a guideline for the design of superior catalysts for important chemical transformation processes.
Impact of the ACS-PRF Award on Students and the PI. This ACS-PRF award has supported several graduate students and three peer-reviewed journal papers has been published. These students learned the fundamental colloidal synthesis methods in preparation well-controlled nanostructures and many characterization techniques, such as transmission electron microscopy, X-ray photoelectron spectroscopy, and Fourier transform infrared spectroscopy (FTIR). They also learned the operation of flow reactors to evaluate catalytic properties of nanomaterials. This knowledge will benefit them for their future career.
This DNI award is also a great support for the PI’s early academic career to develop this new research direction on catalysis using iNPs. Through this support, the PI’s group has acquired critical preliminary results for the application of funds from other federal agencies. This research also brought many interesting collaborations between the PI’s group and other research institutes.