Reports: UR152798-UR1: Synthesis of Azaannulenes Related to the Porphyrins
Timothy D. Lash, Illinois State University
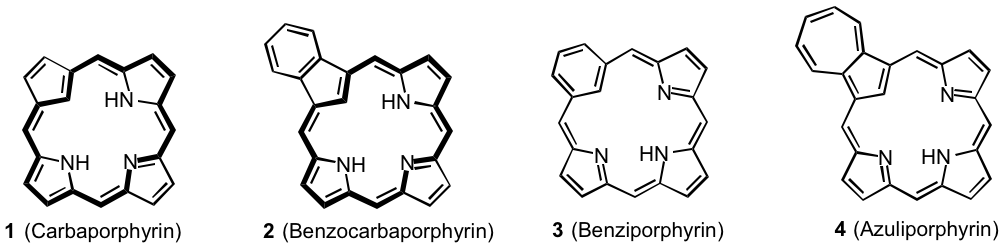
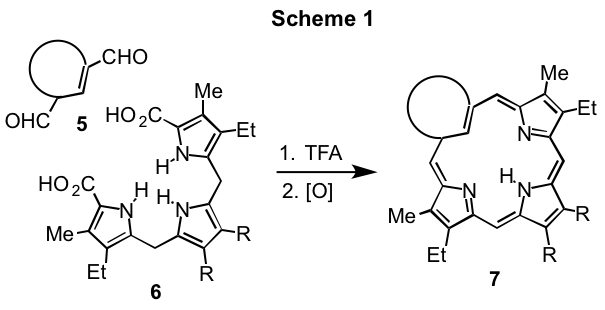
Porphyrins are macrocyclic systems built up from four pyrrolic subunits that possess global aromatic properties. Porphyrin analogues that have one or two of the pyrrole units replaced by carbocyclic rings may retain these aromatic characteristics but in some cases these systems are nonaromatic or even antiaromatic.1 True carbaporphyrins such as 1 and 2 exhibit the strong diamagnetic ring currents associated with aromatic systems,1,2 while benziporphyrins 3 are essentially nonaromatic3 and azuliporphyrins 4 have intermediary properties.4 These systems exhibit unusual reactivity, undergoing selective oxidation reactions and generating stable organometallic derivatives under mild conditions. In addition, derivatives of benzocarbaporphyrins 2 have been shown to be effective agents in the treatment of leishmaniasis.5 Carbaporphyrinoids can be synthesized by a '3 + 1' variant on the MacDonald reaction where an aromatic dialdehyde 5 is condensed with a tripyrrane 6 in the presence of TFA, and following an oxidation step carbaporphyrinoids 7 are often isolated in good yields (Scheme 1). This strategy has been very successful, and has provided straightforward access to carbaporphyrinoids such as 1-4.1 However, it has been necessary to develop alternative strategies to access further modified structures such as dicarbaporphyrinoids.6
In recent investigations, we have developed a '2 + 2' approach to adj-dicarbachlorins. Dicyclopentadienylmethane 8 was reacted with dipyrrylmethane dialdehyde 9 and KOH in refluxing methanol (Scheme 2).7 The best results were obtained when the reaction was carried out for 4 days using excess 8 and a dicarbachlorin product 10 was isolated in 5-7% yield. The UV-vis spectrum for this product was very similar to porphyrins or carbaporphyrins such as 2 in that is showed a strong Soret band at 398 nm and a series of Q bands at 501, 533, 619 and 683 nm. The proton NMR spectrum of 10 in CDCl3 indicated that an asymmetrical porphyrinoid product had been generated with upfield resonances at -6.68 (2H) and -4.03 ppm (1H) for the internal CH2 and CH units, respectively. These results clearly showed that the compound corresponded to 10 rather than tautomer 10' with two internal NHs, and confirmed that the first example of a carbaporphyrinoid system with an internal methylene unit had been generated.7 The dicarbachlorin system represents a new, albeit long anticipated, structural variation for porphyrin analogues and opens up new possibilities for carbaporphyrin research.
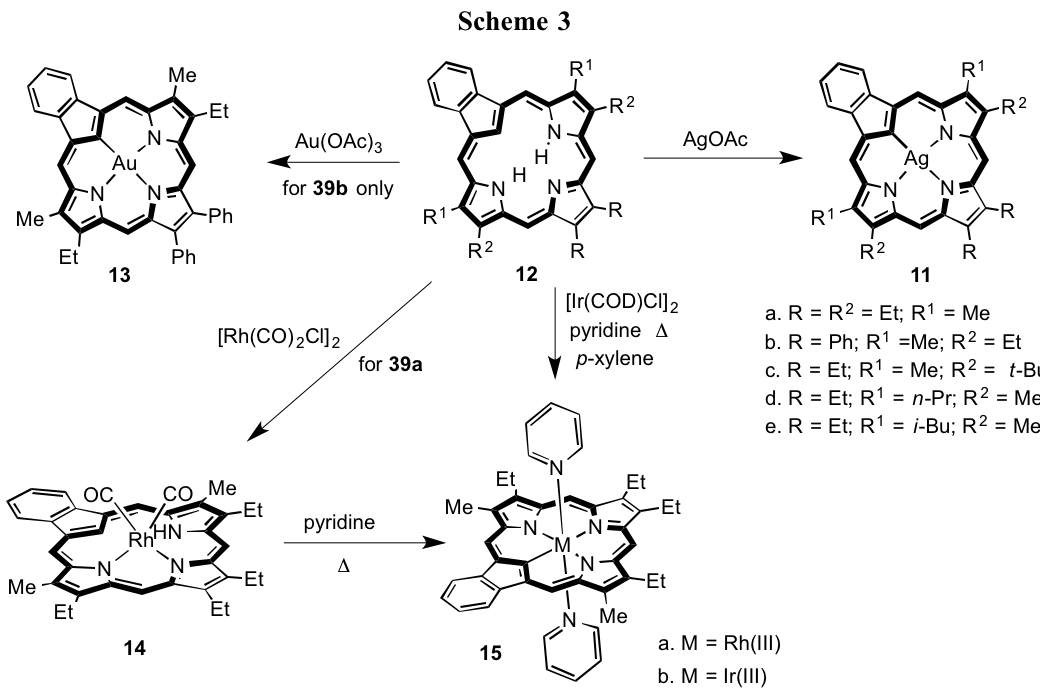
Initial investigations into the metalation of benzocarbaporphyrins were unsuccessful, as all attempts to react these potential ligands with Pd2+, Ni2+, Cu2+, Zn2+ or Co2+ failed to give any identifiable derivatives. Ferric chloride did react but gave carbaporphyrin ketals via an unusual oxidative pathway.8 However, silver(III) complexes 8 were subsequently prepared by reacting benzocarbaporphyrins such as 9 with silver(I) acetate (Scheme 2) and gold(III) complexes such as 10 were also observed. Nevertheless, the organometallic chemistry of benzocarbaporphyrins has not received the attention that it deserves. To address this issue, the formation of rhodium and iridium complexes of 9 have been investigated. Treatment of 9 with 1 equiv. of di-m-chlorotetracarbonyldirhodium(I) gave rhodium(I) complex 11 90% yield (Scheme 2). The proton NMR spectrum in CDCl3 demonstrated that the ligand retained fully aromatic characteristics, and the internal CH gave rise to a resonance at -5.52 ppm, while the NH afforded an upfield peak at -2.54 ppm. When 11 was refluxed in pyridine for 1 h, a deep red colored product was generated corresponding to rhodium(III) complex 12a (Scheme 2). At 50 oC, the proton NMR spectrum showed the meso-protons as two broad peaks at 9.33 and 9.74 ppm and these results imply the presence of a strong diamagnetic ring current. Refluxing 9 with [Ir(COD)Cl]2, pyridine and sodium acetate in refluxing p-xylene gave the corresponding iridium(III) complex 12b in 22% yield (Scheme 2). Again, the proton NMR spectrum of 12b was consistent with a fully aromatic species and the meso-protons appeared as two downfield singlets at 9.40 and 9.83 ppm. The structures of 11, 12a and 12b were confirmed by single crystal X-ray diffraction.
References
1. Lash, T. D. Recent advances in the synthesis and chemistry of carbaporphyrins and related porphyrinoid systems, Eur. J. Org. Chem. 2007, 5461-5481.
2. Li, D.; Lash, T. D. Synthesis and reactivity of carbachlorins and carbaporphyrins. J. Org. Chem. 2014, 79, 7112-7121.
3. Lash, T. D. Benziporphyrins, a unique platform for exploring the aromatic characteristics of porphyrinoid systems, Org. Biomol. Chem. 2015, 13, 7846-7878.
4. Lash, T. D. Out of the blue! Azuliporphyrins and related carbaporphyrinoid systems Acc. Chem. Res. 2016, 49, 471-482.
5. Taylor, V. M.; Cede–o, D. L.; Mu–oz, D. L.; Jones, M. A.; Lash, T. D.; Young, A. M.; Constantino, M. H.; Esposito, N.; VŽlez, I. D.; Robledo, S. M. In Vivo and In Vitro Studies of the Utility of Dimethyl and Diethyl Carbaporphyrin ketals in the Treatment of Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2011, 55, 4755-4764.
6. AbuSalim, D. I.; Ferrence, G. M.; Lash, T. D. Synthesis of an adj-dicarbaporphyrin and the formation of an unprecedented tripalladium sandwich complex. J. Am. Chem. Soc. 2014, 136, 6763-6772.
7. Lash, T. D.; AbuSalim, D. I.; Ferrence, G. M. adj-Dicarbachlorin, the first example of a free base carbaporphyrinoid system with an internal methylene unit. Chem. Commun. 2015, 51, 15952-15955.
8. Lash, T. D.; Muckey, M. A.; Hayes, M. J.; Liu, D.; Spence, J. D.; Ferrence, G. M. Regioselective Oxidation of Benzocarbaporphyrins with Ferric Chloride: A Facile Synthesis of Bridged [18]Annulene Ketals with Strong Absorptions in the Far Red and an Unexpected Halogenation Reaction at the Interior Carbon Atom. J. Org. Chem. 2003, 68, 8558-8570.
9. Lash, T. D.; Colby, D. A.; Szczepura, L. F. New Riches in Carbaporphyrin Chemistry: Silver and Gold Organometallic Complexes of Benzocarbaporphyrins. Inorg. Chem. 2004, 43, 5258-5267.
10. Adiraju, V. A. K.; Ferrence, G. M.; Lash, T. D. Rhodium(I), rhodium(III) and iridium(III) carbaporphyrins. Dalton Trans. 2016, 45, 13691 - 13694