Reports: UR354825-UR3: Phosphine-Directed C-H Borylation of Arenes: Facile Access to Ambiphilic Phosphine Boronate Esters
Timothy B. Clark, PhD, University of San Diego
Technical Report:
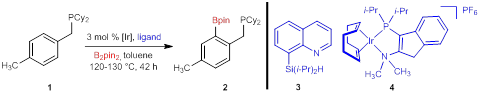
The first aim of the proposal was to identify reaction conditions that were more general for the phosphine-directed C–H borylation of phosphines. In order to develop reaction conditions that are amenable to a wider range of phosphine substrates, a new catalyst system was sought with increased activity and selectivity for mono-ortho borylation. Several existing and newly-designed catalyst systems were examined with benzylic phosphines. Two particular catalysts provided improved reactivity: the silylquinoline ligand (3) reported by Smith and iridium cationic complex 4 (Scheme 1). While the catalyst system with ligand 3 provided increased conversion to the borylation product, the selectivity for mono ortho-borylation product was diminished.
Scheme 1. Catalyst Reactivity for Phosphine-Directed C–H Borylation
[Ir] Catalyst/Ligand | Conv. | mono:bis |
[Ir(COD)OMe]2 / no ligand | 58% | 98:2 |
[Ir(COD)OMe]2 / 6 mol % 3 | 93% | 80:20 |
4 / no ligand | 94% | 90:10 |
The unexpected high reactivity of cationic complex 4 with phosphine 1 led us to screen other cationic iridium complexes with rigid, electron-rich bidentate ligands that are commonly used for non-directed C–H borylation.96 The combination of [(COD)2Ir]BF4 and TMPHEN was found to be nearly unreactive with B2pin2 (Scheme 2), but highly reactive with pinacolborane (HBpin). The equivalents of HBpin used and the reaction temperature and time was optimized, allowing the reaction of phosphine 5 to occur in 18 h at 110 °C, which was unreactive using the our previous reaction conditions.
Scheme 2.
Optimization and Substrate Scope of Phosphine-Directed C–H Borylation
Boron (equiv) | Temp (oC) | t (h) | Conv. | Select. |
B2pin2 (1.2) | 130 | 24 | <20% | >99:1 |
HBpin (8) | 130 | 24 | 99% | 87:13 |
HBpin (4) | 120 | 18 | 97% | >99:1 |
HBpin (4) | 110 | 18 | 99% | >99:1 |
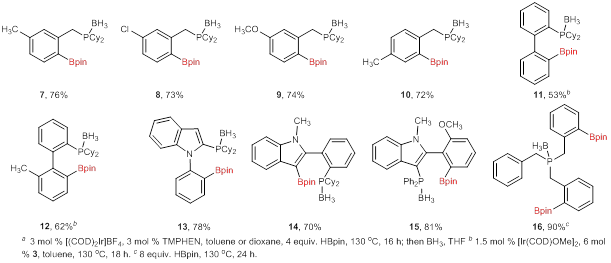
Multiple phosphines have been examined under these optimized reaction conditions, providing a variety of phosphine boronate esters in moderate to good yield upon protection as the borane complex (Figure 1). Borylated benzylic phosphines were isolated in consistently high yields (7–10). Biphenylphosphines were not reactive under the optimized reaction conditions, but were found reactive when silylquinoline (3, Scheme 1) was used as the ligand. Indole-based phosphines provided phosphine boronates 13–15 in high yields as well. Finally, tribenzylphosphine borylation was optimized to provide bisborylated phosphine 16 in 90% yield. Conditions have also been identified to provide the mono and tris-borylation products as the major compound, but isolation of these products is still underway.
Figure 1. Scope of C–H Borylation with Optimized Conditionsa
The use of a cationic iridium complex in C–H borylation has not been reported in the literature to the best of our knowledge. In fact, the use of a cationic complex is opposite to the generally accepted lore that electron rich iridium complexes are ideal for increased reactivity in C–H borylation.
The cationic nature of the iridium complex, however, does meet the criteria for substrate-directed C–H borylation by providing an additional open coordination site by replacing one Bpin ligand with a non-coordinating counter-ion. This new approach to directed C–H borylation is expected to provide unique reactivity for a variety of Lewis basic directing groups in addition to phosphines.
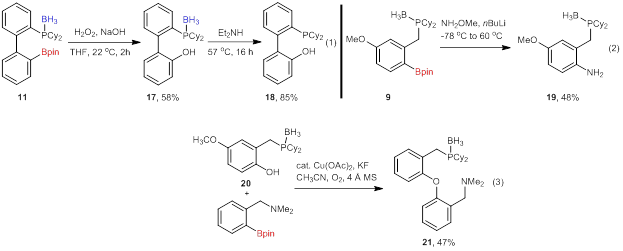
Early progress has also been made on developing reactions that utilize the newly formed C–B bond in synthesis. The oxidation and amination of the C–B bond has been achieved to access protected phosphinophenols (17, eq 1) and aminophosphines (19, eq 2). The deprotection of the phosphine in 17 also provided the corresponding phosphinophenol (18) in high yield. The amination reaction requires optimization to improve the reaction yield, but both reactions will be applied to a variety of protected phosphine boronates (such as those in Figure 1) to determine the scope of the reaction. The final transformation that we have achieved is the Chan-Evans-Lam coupling of a phenol to an aminoboronate ester (accessed from another project) to provide 21. This reaction also requires optimization to improve the yield. We anticipate access to this class of aminophosphines will result in a new type of pincer ligand that may have distinct reactivity from the wide array of pincers that are currently known.
Development of new conditions for phosphine-directed C–H borylation is a critical step to the success of this project, allowing reliable access to phosphine boronate esters that can be used in a number of transformations. This grant has provided the needed funding to get this project from an early new direction, to a promising project that should garner additional external support. This project is projected as a main area of investigation for the PI in the coming years and will be instrumental in his continued success. Publication of these results is expected in a top-tier journal during the next granting period.