Reports: ND1054793-ND10: Study of Transition Metal Chalcogenide Nanostructures as Efficient Oxygen Reduction Catalysts
Manashi Nath, PhD, Missouri University of Science and Technology
Transition Metal Selenides as High Efficiency Electrocatalyst for Oxygen Evolution Reaction (OER) in Alkaline Medium
Nath group have identified several high efficiency selenide based electrocatalysts for oxygen evolution reaction (OER) in alkaline medium, each exhibiting very low overpotential at 10 mA/cm2, viz. Ni3Se2 (290mV), NiSe2 (140mV), NiFe2Se4 (210mV), FeNi2Se4 (160mV), NiCo2Se4 (170mV), Co7Se8 (260mV), Ni2Te3 (160mV), Ni1-xCoxFe2Se4 (220mV), Cu2Se (320mV), and a molecular seleno-based Ni coordination complex. Some of these studies has been outlined below:
NiSe2: Highly Efficient Bifunctional Electrocatalyst for Water Splitting and the Importance of Preferred Lattice Orientation
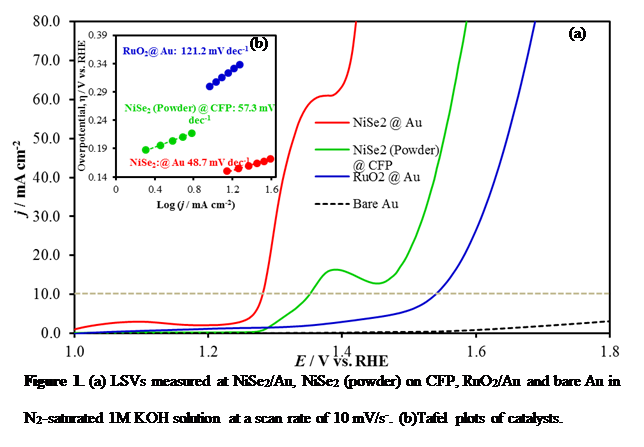
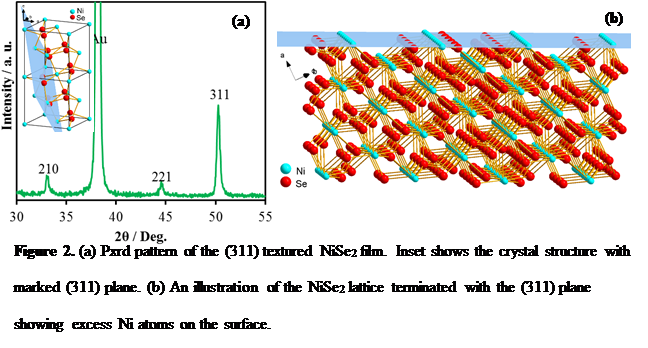
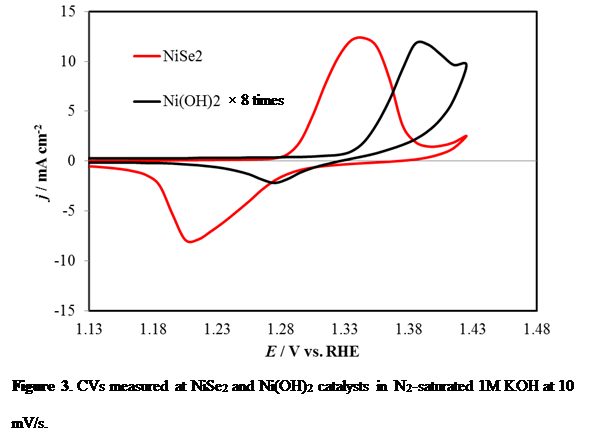
Herein we have shown that electrodeposited NiSe2 can be used as a bifunctional electrocatalyst under alkaline conditions to split water at very low potential by catalyzing both oxygen evolution and hydrogen evolution reactions at anode and cathode, respectively, achieving a very high electrolysis energy efficiency exceeding 80% at considerably high current densities (100 mA/cm). The OER catalytic activity as well as electrolysis energy efficiency surpasses any previously reported OER electrocatalyst in alkaline medium and energy efficiency of an electrolyzer using state-of-the-art Pt and RuO2 as the HER and OER catalyst, respectively. Specifically, for OER it produces 10 mA/cm2 at an overpotential of 140mV, which is the lowest that has been reported till date (Figure 1). Through detailed electrochemical and structural characterization, we have shown that the enhanced catalytic activity is attributed to directional growth of the electrodeposited film that exposes a Ni-rich <311> lattice plane as the terminating plane (Figure 2). The effect of increased covalency of the selenide lattice was also visible as it lowered the Ni2+ to Ni3+ oxidation potential (Figure 3), thereby facilitating the generation of Ni3+ which is the actual catalytic species.
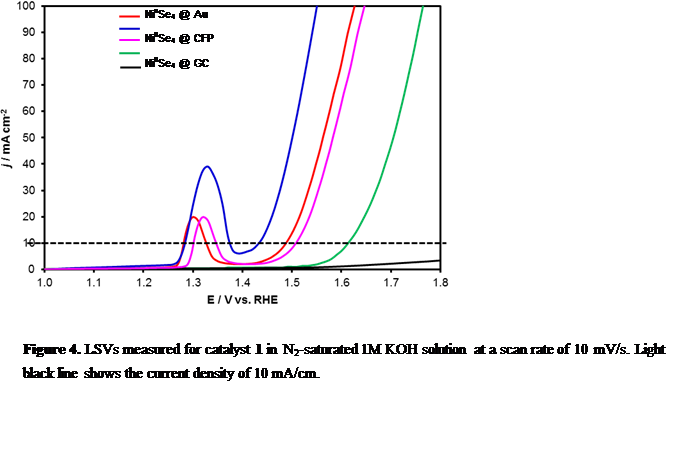
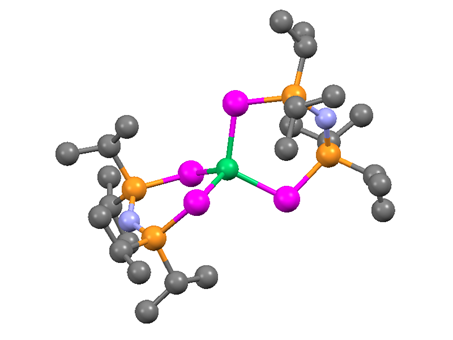
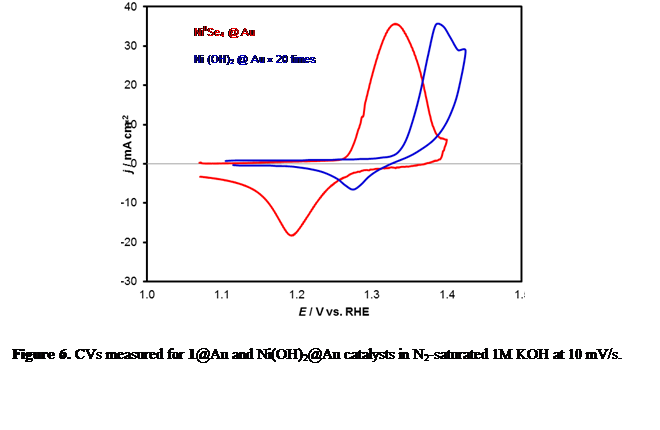
Effect of Increasing Covalency: Our primary hypothesis was that the catalytic efficiency for OER can be enhanced by increasing covalency in the lattice. Thus compared to the oxides, the selenides are expected to have better catalytic activity, since Se is less electronegative than O, therefore, increasing the degree of covalency in the metal-chalcogen bond. Recently, we have generated some proof for this hypothesis by studying the catalytic activity of a seleno-based molecular precursor, [Ni{(SePiPr2)2N}2] (1) containing a tetrahedral NiIISe4 core (Figure 4) which exhibits an excellent OER activity in 1M KOH with a small overpotential of 200mV at 10mA/cm2 (Figure 5). This complex is also active for HER in alkaline medium showing overpotentials of 330mV to achieve 10mA/cm2 for HER. The overpotential for OER is one of the lowest that has been reported till date, making this one of the best OER electrocatalysts. In addition, this molecular complex exhibits an exceptionally high mass activity (111.02A/g) and a much higher TOF value (0.26 s-1) at a overpotential of 300mV. This bifunctional electrocatalyst enables water electrolysis in alkaline solutions at a cell voltage of 1.54V. The enhanced activity for OER is attributed to the increased covalency of the metal-chalcogen bond which decreases the Ni2+ to Ni3+ oxidation potential compared to that of the oxides thereby enhancing catalyst performance (Figure 6).
Effect of Transition Metal Doping: Mixed metal selenides as efficient OER catalysts
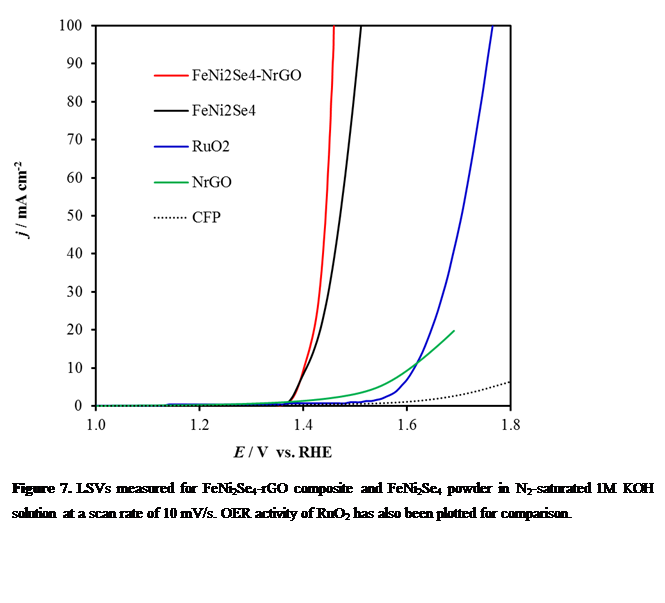
FeNi2Se4–rGO composite: We have synthesized a hybrid material FeNi2Se4 supported on nitrogen doped reduced graphene oxide (FeNi2Se4-NRGO) as an efficient and dependable electrocatalyst for oxygen evolution reaction (OER) under alkaline conditions. The constructed hybrid catalyst is capable of catalyzing water oxidation at a small overpotential of 210 mV, compared to FeNi2Se4 or nitrogen doped reduced graphene oxide alone which has little catalytic activity (Figure 7). This is possibly due to the synergistic chemical coupling effects between the FeNi2Se4 and nitrogen doped reduced graphene oxide matrix. Chronoamperometric studies shows that the catalyst is stable up to 12h with very less degradation. As a comparison we also synthesized FeNi2O4–NrGO which shows an overpotential of 310mV to achieve the current density of 10 mA/cm2.
Advancing State of the Art with respect to OER electrocatalysts:
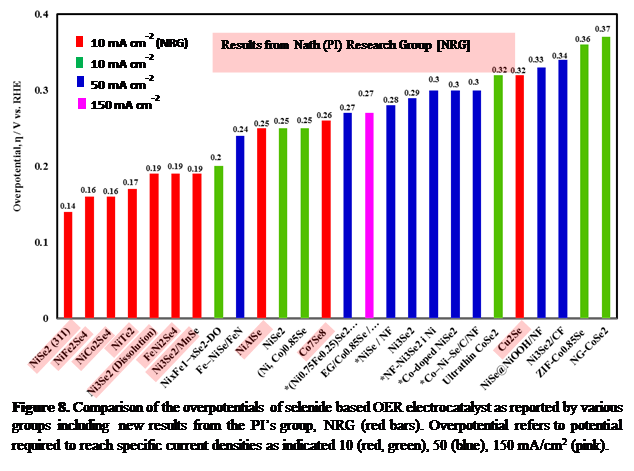
Through our persistent efforts we were able to lower the overpotential for oxygen evolution from alkaline order by several orders of magnitude compared to other transition metal selenides or the conventional precious metal and transition metal oxides (Figure 8). This is indeed remarkable especially in current times, when clean affordable energy generation without depleting earth’s natural resources or mineral reserves, has been one of the prime concerns. Replacing precious metals with earth-abundant elements along with increasing catalytic efficiency will definitely boost the advancement of water-splitting technology.
Figure 2. (a) Pxrd pattern of the (311) textured NiSe2 film. Inset shows the crystal structure with marked (311) plane. (b) An illustration of the NiSe2 lattice terminated with the (311) plane showing excess Ni atoms on the surface.
(a)
(b)
Figure 3. CVs measured at NiSe2 and Ni(OH)2 catalysts in N2-saturated 1M KOH at 10 mV/s.
× 8 times
Figure 4. LSVs measured for catalyst 1 in N2-saturated 1M KOH solution at a scan rate of 10 mV/s. Light black line shows the current density of 10 mA/cm.
NiIISe4 @ Au
NiIISe4 @ CFP
NiIISe4 @ GC
RuO2 @ Au
Bare Au
Figure 5. Molecular structure of [Ni{(SePiPr2)2N}2] showing the tetrahedral seleno-coordination of NiII. Ni-green, Se-magenta, P-brown.
Figure 6. CVs measured for 1@Au and Ni(OH)2@Au catalysts in N2-saturated 1M KOH at 10 mV/s.
NiIISe4 @ Au
Ni (OH)2 @ Au × 20 times
Figure 7. LSVs measured for FeNi2Se4-rGO composite and FeNi2Se4 powder in N2-saturated 1M KOH solution at a scan rate of 10 mV/s. OER activity of RuO2 has also been plotted for comparison.
10 mA cm-2 (NRG)
10 mA cm-2
50 mA cm-2
150 mA cm-2
Figure 8. Comparison of the overpotentials of selenide based OER electrocatalyst as reported by various groups including new results from the PI’s group, NRG (red bars). Overpotential refers to potential required to reach specific current densities as indicated 10 (red, green), 50 (blue), 150 mA/cm2 (pink).
Results from Nath (PI) Research Group [NRG]