Reports: UNI454810-UNI4: Structure-Oxidation Potential Relationship of Antioxidants and Peroxide Formation in Gasoline
Sanela Martic, PhD, Oakland University
Antioxidants are commonly used as gasoline additives in order to improve fuel efficiency and minimize the deterioration of the automotive parts. However, the gasoline composition directly affects its physical properties and engine performance, both of which may compromise vehicle integrity and durability. With the support from the ACS PRF, my lab proposed to 1) establish a structure-function relationship of antioxidants based on the butylated phenol core toward identifying key features of “super-antioxidants”, 2) determine antioxidant activity of butylated phenols, and 3) characterize butylated phenol additives in gasoline samples.
Since being awarded PRF support in Aug 2015, my group has been working on fully characterizing redox properties of a series of butylated phenols by electrochemical means. We have serendipitously discovered the color switching, electrochromism, by 2,4-di-tert-butylphenol (DTBP) (Fig. 1A-B). This finding had triggered detailed structural and electronic investigations of oxidation of bulky phenols towards understanding the mechanism of electrochromism. The electrochemical and spectroscopic studies revealed that the stability and reactivity of radicals, alongside the steric hindrance dictated the electrochromic outcome as induced by the electrochemical oxidation. The electrochromism produced a major biphenylquinone product which was isolated and characterized by X-ray diffraction. By contrast, the chemical oxidation of bulky phenols was not a selective process, and the electrochromism was not unique to 2,4-di-tert-butylphenol (DTBP) under the chemical conditions. The results on the selective electrochromism of selective bulky phenol indicated that this class of antioxidants may have additional applications in material design. This work has been published in the ACS J. Phys. Chem. B in 2016.
Fig. 1 (A) Digital photos and (B) UV-vis spectra of 2,4-di-tert-butylphenol (DTBP) clear solution before electrochemical cycling and after electrochemical cycling (yellow solution).
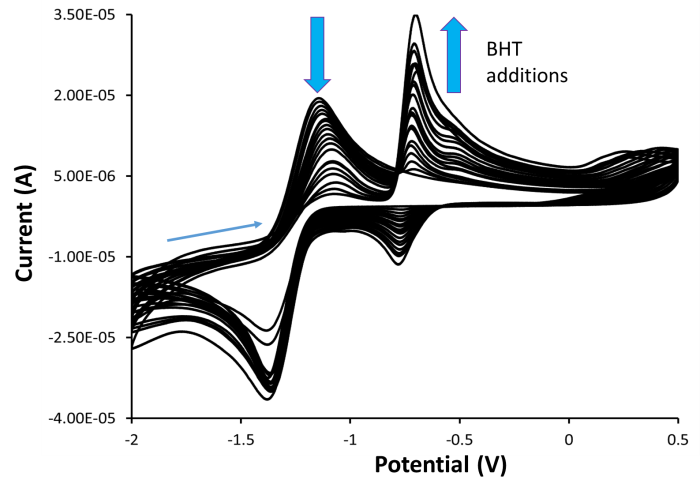
The second project is aimed at determining the antioxidant activity of bulky phenols with superoxide and hydrogen peroxide by using a very sensitive analytical electrochemical method. The superoxide and hydrogen peroxide levels were measured by electrochemical means in the absence and presence of antioxidants. Our unpublished data indicated that bulky phenols, such as the 2,6-di-tert-butyl-4-methylphenol (BHT), may act as antioxidants (Fig. 2). Currently, we are evaluating and comparing these antioxidants in terms of their ability to react with hydrogen peroxide. We aim to determine how a substitution pattern around a phenol ring affects the antioxidant activity.
Fig. 2 Cyclic voltamograms of 0.1 M TBAP in anhydrous DMF with sequential additions of 2,6-di-tert-butyl-4-methylphenol (BHT).
As a part of this funding, the third project is aimed at characterizing commercial gasoline samples using the electrochemical methods. This will allow for electrochemical screening of fuel content as well as determination of electrochemical parameters, such as capacitance and resistance of the gasoline.
This PRF award was used to support two undergraduate students (Sep 2015-Oct 2016 and Jan 2016-April 2016). Currently one undergraduate and one high school students are involved in the projects mentioned. Based on this work, the students presented 4 poster presentations at various meetings (regional Meeting or Minds, institutional student symposium and research festival, regional physical-organic mini symposium). The undergraduate student also presented an oral presentation at the national 251st ACS meeting in San Diego. The research data acquired within the first year of funding were also disseminated in the form of the peer-reviewed publication in the ACS J. Phys. Chem. B (2016). One other manuscript, based on this work, is currently in preparation. PRF funding has given the undergraduates greater research opportunities, and has certainly increased research productivity in my own laboratory. The funding has also allowed me to expand my research scope and interests.