Reports: ND553404-ND5: Effects of Ions on Interfacial Tension and Electrical Double Layer
Zhen-Gang Wang, California Institute of Technology
Research Accomplishments and Scientific Significance
Our efforts during the last reporting period focused on extending the dipolar self-consistent field theory for nonequilibrium charge solvation in pure liquids to liquid mixtures in the context of electron transfer reactions.
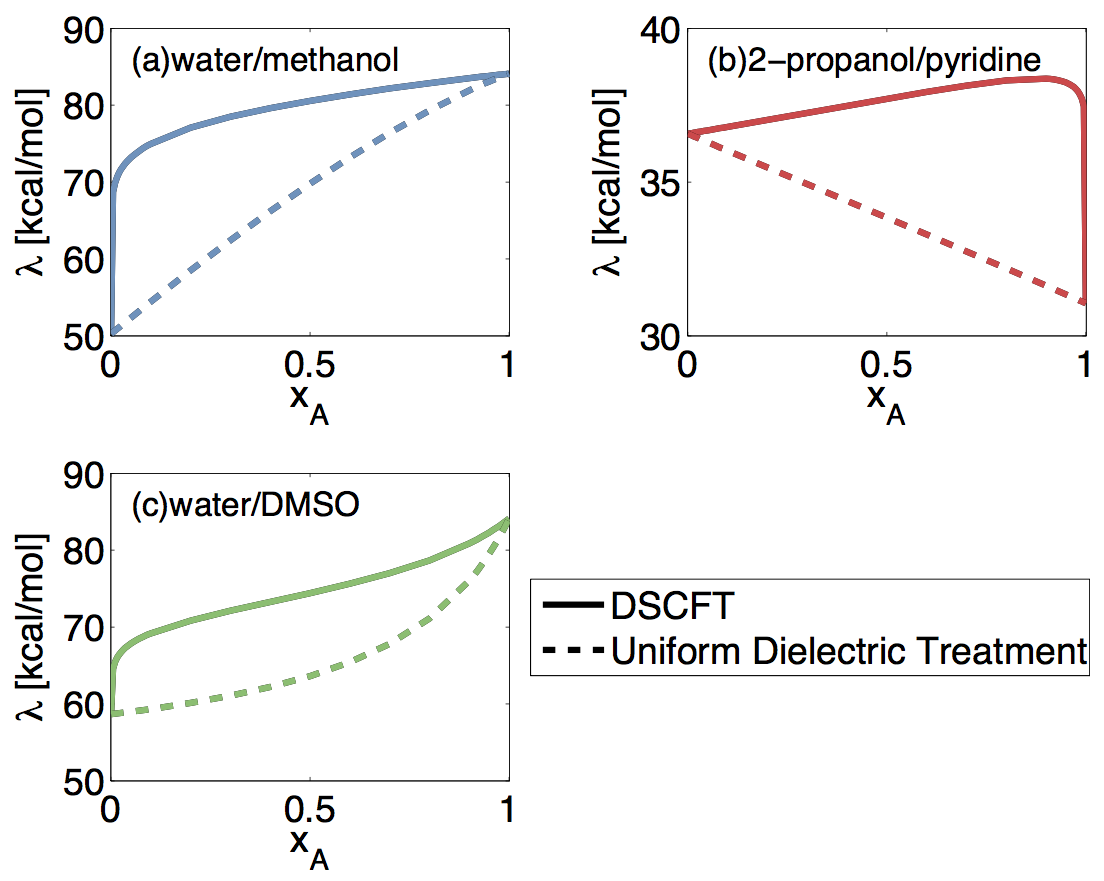
Using statistical field techniques, we have developed a coarse-grained theory for charge solvation in liquid mixtures under equilibrium and nonequilibrium conditions, and apply it to compute the solvent reorganization energy of electron transfer (ET) reactions. Using molecular parameters that are readily available, the DSCFT naturally accounts for the dielectric saturation effect and the spatially varying solvent composition in the vicinity of the reacting species. Our work shows that, in addition to the nonequilibrium orientational polarization, the reorganization energy in liquid mixtures depends strongly on the out-of-equilibrium solvent composition around the reacting species due to preferential solvation. We identify three general categories of binary solvent mixtures; each category is shown to produce a characteristic local solvent composition profile around the reacting species, giving rise to a distinctive composition dependence of the reorganization energy that cannot be predicted using the bulk dielectric constants of the solvent mixtures; see Fig. 1.
Figure 1. Solvent reorganization energy vs. the mole fraction of component A, for electron self-exchange reaction Ag1++Ag0 → Ag0+ Ag1+ in (a) water/methanol, (b) 2-propanol/pyridine, and (c) water/DMSO mixtures. The component A refers to the first component (component before the /). The solid curve are results calculated with the DSCFT, while the dashed line are results calculated from the uniform bulk dielectric treatment.
Electron transfer is ubiquitous in many chemical and biological processes, and it has long been established that solvent fluctuation plays a central role in the kinetics and dynamics of ET processes. The pioneering work by Rudolph A. Marcus in 1956 provided a clear mechanistic picture for the process, and subsequent applications and extensions of the theory continue to the present day. However, the effects of mixed solvents on ET remain largely unexplored, despite the common usage of mixture as electrolytes in industrial applications. Existing experimental studies have shown distinctive behaviors in the ET rates depending on the specific redox species and the mixture components, but no systematic trend has emerged to allow for predictive understanding of the behaviors in general mixtures. To the best of our knowledge, our theory is the first attempt at systematically elucidating the connection between the reorganization energy and local solvent composition around the reacting species for ET processes in mixed solvents, with both the solvent nuclear and electronic degrees of freedom accounted for. The work addresses a fundamental question in electron-transfer reactions, and is relevant to a wide range of systems of current interest, including batteries and charge-transfer biomolecular systems.