Reports: DNI755725-DNI7: Nanoemulsion-Templated Porous Polymers
Reza Foudazi, PhD, New Mexico State University
Kinetically stable emulsions are formed under extreme shears in the size range of 50-200 nm, which are called as nanoemulsions. One of the applications of nanoemulsions is to use them as templates for making mesoporous polymers. The templates of High Internal Phase Emulsions (HIPEs) and mesophases of surfactant self-assembly have been used to produce porous polymers with pore sizes bigger than 1 μm and smaller than 10 nm, respectively. However, novel methods are required to fill the current pore-size gap (10nm-500nm) between mesoporous and macroporous polymers. Template of nanoemulsions can be a good candidate to fill up the gap between mesoporous and macroporous polymers.
2. Experimental
Four different surfactants with different polarities are used: anionic surfactant sodium dodecyl sulfate (SDS), cationic surfactants dodecyltrimethylammonium bromide (DTAB) and cetyltrimethylammonium bromide (CTAB), and non-ionic surfactant Pluronic F68. Silicone oil with density of 0.914 g/cm3 was used as the dispersed phase, while deionized water was used as the continuous phase. For the crosslinking polymerization process, 2-hydroxyethyl methacrylate (HEMA), poly(ethylene glycol) diacrylate (PEG-DA), and potassium persulfate (KPS) were used as the monomer, the cross-linker, and the thermal initiator, respectively.
Oil-in-water nanoemulsions were prepared by using a high energy ultrasonic processor. Then, the continuous phase was partially extracted from emulsions by applying gravitational force using centrifugation at 11,000 rpm for several hours at room temperature. Consequently, droplets were concentrated into a highly elastic cream at the top of the centrifuge tube since the density of the silicone oil is less than that of water. Then, the cream was diluted in a solution contained deionized water (56 wt.%), 2-hydroxyethyl methacrylate (HEMA) (33 wt.%), PEG-DA (10 wt.%), and potassium persulfate (KPS) (1 wt.%) for polymerization.
Dynamic light scattering (λ=633nm, θ=173o) was carried out in a Zetasizer (Malvern Instruments, UK) at 25oC after dilution of samples in deionized water. The polydispersity index (PDI) was obtained from the broadness of the distribution, scaled in the range from 0 to1.
3. Results and Discussion
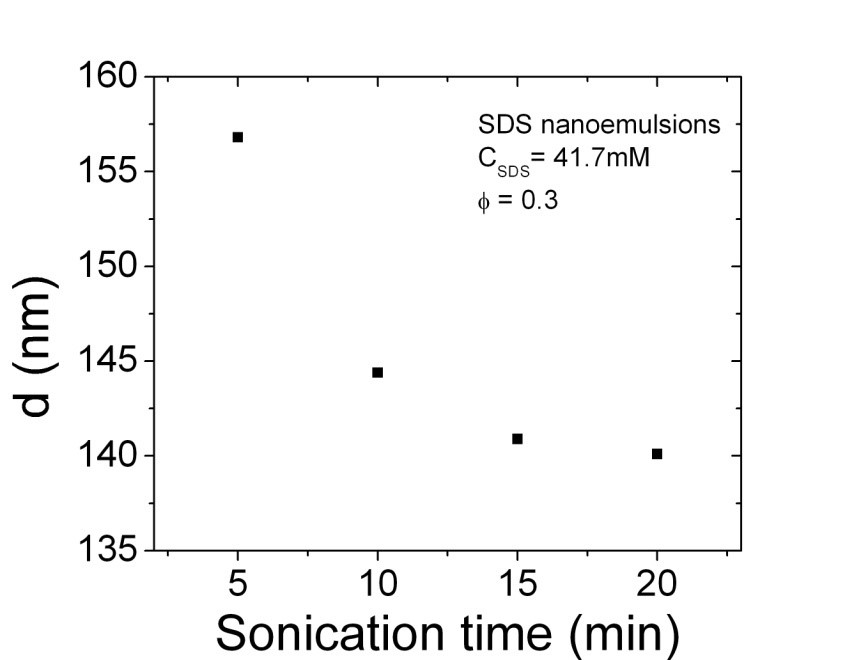
The droplets size decreases with increasing the sonication time up to a plateau, as typically shown in Figure 1. The internal phase volume fraction was varied from 0.2 to 0.45 at different surfactant concentration. The surfactant concentration was varied from 2*CMC to 50*CMC. It is clearly evident from the DLS data that the increasing of surfactants concentration reduces the droplets size significantly.
Figure 1: Droplet size change of SDS nanoemulsions with sonication time.
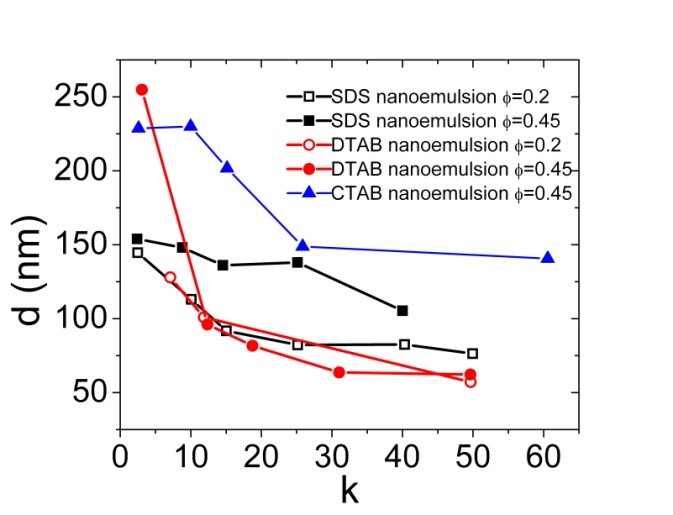
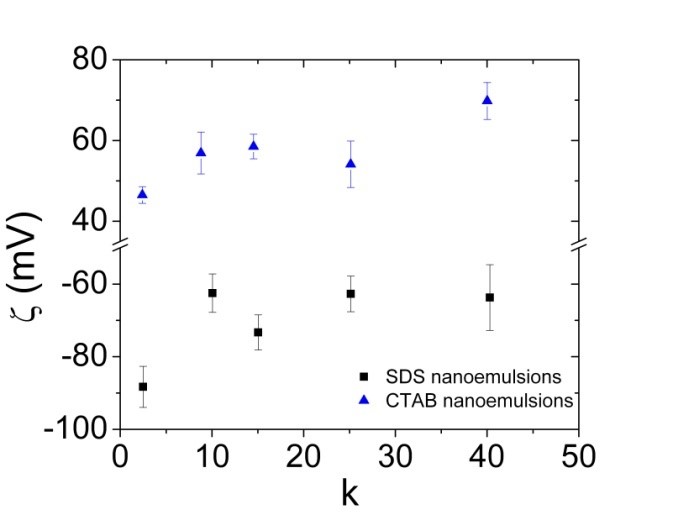
At f=0.45, the nanoemulsions containing SDS surfactant is yielded droplets size from 100nm to 160nm for the amphiphile concentration of 19mM to 330mM. The similar effect is observed for the nanoemulsions containing CTAB, DTAB and F68, while nanodroplets prepared by using F68 surfactant are highly unstable. The stability of nanoemulsions containing different surfactants is determined by ζ-potential. ζ-potential measurements revealed highly positive charges for DTAB and CTAB stabilized nanodroplets, while highly negative charges for SDS stabilized nanodroplets. ζ-potential for F68 stabilized nanodroplets is close to zero which explains their very low stability. The size measurement of nanoemulsions at different concentration and different internal phase volume fraction is shown in Figure 2, where the x-axis is denoted as “k”, signifying the multiplier of the CMC of the corresponding surfactants. It is observed that the droplet size of nanoemulsions is decreased slightly for DTAB and significantly for SDS with decreasing the internal phase volume fraction while keeping the surfactant concentration constant. The nanoemulsion corresponding to the first data point for DTAB at f=0.2 did not show adequate stability over time. In summary, by decreasing the volume fraction and increasing the surfactant concentration, optically transparent nanoemulsions are achieved. The polydispersity index for most of these nanoemulsions are in the 0.1 to 0.16 range.
Figure 2: (Left) Droplet size variation of nanoemulsions at different SDS, CTAB and DTAB concentrations and different internal phase volume fractions. (Right) ζ-potential of nanoemulsions at different SDS and CTAB concentrations.
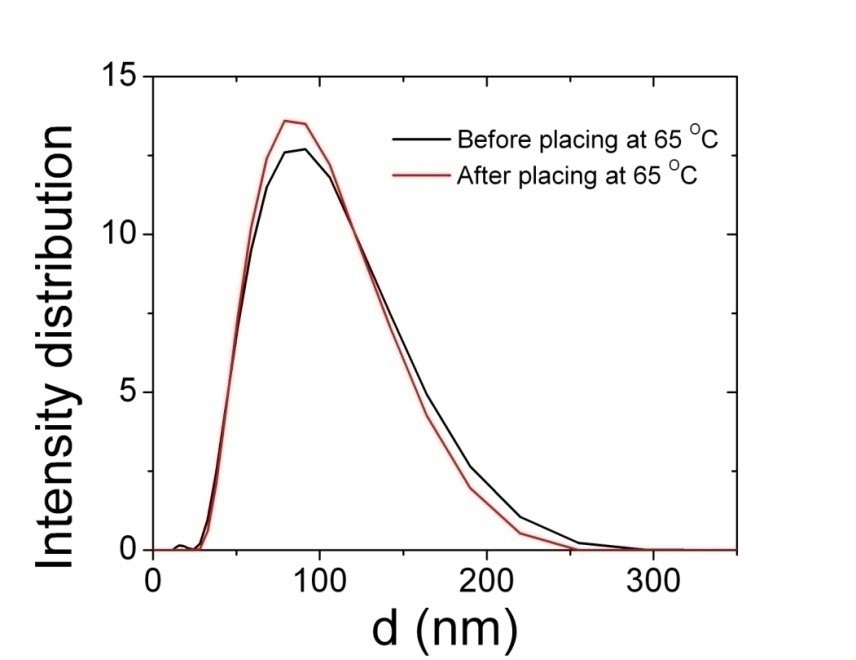
The temperature stability of nanoemulsions droplets was tested by DLS measurement. The cream extracted from nanoemulsions was placed in an oven at 65oC for 6 hours. Very close DLS data (Figure 3) before and after heat treatment of cream shows that these nanoemulsions are suitable for thermal initiator triggered polymerization.
Figure 3: Temperature stability of SDS contained nanoemulsions before and after heat treatment.
The nanoemulsion creams were diluted in a solution contained DI water, HEMA, PEG-DA, and KPS for polymerization. This step yielded the breaking of nanoemulsions into two phases. The oil phase was stayed at the top while the water phase remained in the bottom with the monomer and the cross-linker. In order to confirm the complete phase separation, nanoemulsions were centrifuged for 20min at 11,000rpm. The current challenge is to produce nanoemulsions that remain stable after exchanging the continuous phase with polymerizable species.
4. Conclusion Future work
We have produced significantly monodispersed droplets through ultrasonication without using the fractionation process. Nanoemulsions droplets are normally stabilized by the electrostatic repulsions as shown by the ζ-potential measurements. The nanoemulsions have good thermal stability to be utilized for polymerization with thermal initiators. However, when the monomer and the cross-linker are added to the nanoemulsions, the solvent polarity decreases, and thus, the stability decreases according to the DLVO theory. This step leads to breaking of nanoemulsions into two phases – oil phase at the top and aqueous phase containing monomers at the bottom.
5. The Impact of Research
The funding from DNI program of ACS PRF has enabled the PI to expand his prior expertise on emulsion science to nanoemulsions. The practical challenges raised during course of research have broadened the original concept of gravitation ordering to a new direction of electromagnetic-filed ordering, which is under investigation in PI’s research group.
Students have become involved in multidisciplinary research related to petroleum industry, and colloid and polymer science. Additionally, one of the undergrad students and the postdoc fellow involved in this project have had the opportunity to travel to CINT in LANL to perform preliminary Small Angle X-ray Scattering (SAXS) on samples, which provided them hands-on experience on scattering techniques.