Reports: DNI155732-DNI1: Hybrid Lewis Acid/Lewis Base Catalysts for Asymmetric Carbon-Carbon Bond Formation
Chris Dockendorff, PhD, Marquette University
Overview
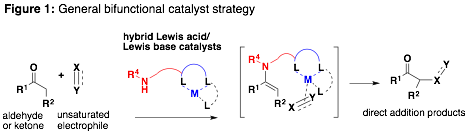
The catalysis arm of our research program is supported by this ACS PRF DNI grant presently focused on the discovery of bifunctional catalysts for the direct addition of aldehydes and ketones to alkynes. Additionally, this grant supported the completion of our early efforts towards the development of new asymmetric aldol catalysts. Our general strategy is outlined in Fig. 1.
Y1 Results
1. Synthesis of azole–carboxamide precatalyst library
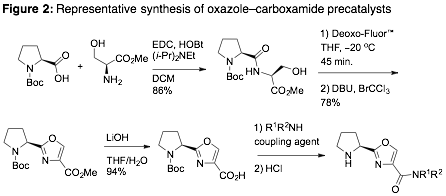
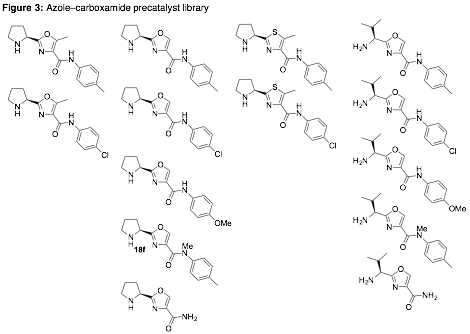
With the synthesis of many members of a proposed azole–oxazoline precatalyst library complete prior to receipt of this grant [1], we focused instead on building new azole–carboxamide-based precatalysts with appropriate geometries for both activating aldehyde/ketone donors, as well as chelating Lewis acids for activation of acceptors (e.g. aldehydes). A representative synthesis is provided in Fig. 2, with the library of oxazole– and thiazole-carboxamide-based precatalysts summarized in Fig. 3 [2].
2. Catalysts for asymmetric direct aldol reactions
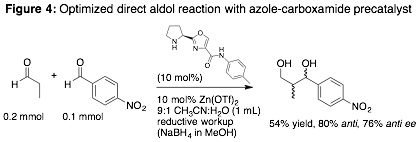
Precatalysts from Fig. 3 were combined with a set of Lewis acidic metal salts and screened in several different aldol reactions [3]. Proline-derived oxazole–carboxamides gave optimal results for the direct addition of propionaldehyde to 4-nitrophenylbenzaldehyde (Fig. 4). Zn(OTf)2 with a mixture of CH3CN and water as solvent gave the best compromise of reactivity and enantioselectivity, and performed similarly to proline as catalyst. Control reactions indicated that the secondary amine moiety needs to be tethered to the oxazole-carboxamide for significant reaction to occur. Disappointingly, these catalysts gave at best sluggish reactions with ketone donors and less-activated aldehyde acceptors. 1H NMR studies suggest that the secondary amine may act as a reversible bridging ligand between metal centers; our current work in this area aims to prevent this phenomenon using solid-supported precatalysts.
4. Preliminary studies for intermolecular additions of aldehydes/ketones to alkynes
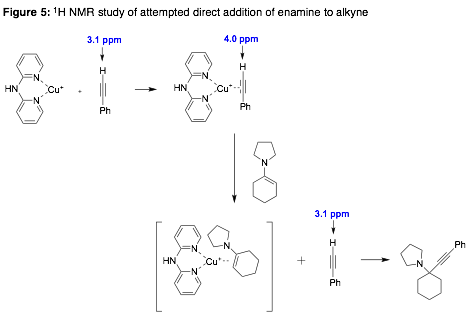
Dual catalyst systems (amines together with transition metals and ligands) have been used to promote intramolecular additions of aldehydes/ketones to unactivated alkynes (i.e. Conia-ene type reactions), but there are very few reports of such intermolecular additions, and we are unaware of enantioselective examples. We hypothesized that activation of an aldehyde/ketone in the form of an enamine intermediate could preclude alkyne–metal binding and activation, since we expect that enamines could be excellent ligands for the pi-acid metals of interest under typical intermolecular conditions. Support for this hypothesis was obtained using cyclohexylamine/Cu(I) catalyst systems that we showed to be effective for Conia-ene type reactions (results not shown), and were modified from those previously reported by Michelet [4]. Addition of a preformed enamine to a CuBF4–alkyne complex led to displacement of the alkyne, and addition of the alkyne to an intermediate iminium ion, a reaction recently reported by Ma [5]. This suggests that a dual catalyst system will be ineffective, and provides justification for the development of a 'pseudo-intramolecular' reaction promoted by a bifunctional catalyst. However, it should be noted that Nakamura has reported In(III)-catalyzed intermolecular additions of stabilized enamines to alkynes, which requires a separate enamine hydrolysis step [6].
5. Design and computational studies of putative catalysts for additions to alkynes
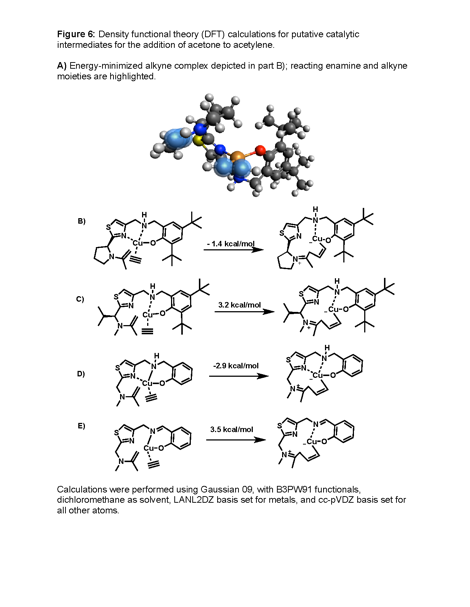
The challenge of bifunctional, hybrid Lewis acid/Lewis base catalysis is that the catalytic acid and base moieties must be situated close enough to bring the nucleophile and electrophile into range for carbon–carbon bond formation, but they cannot be too close, which leads to self-quenching. The synthesis of new precatalysts is usually the most challenging, time-consuming, and costly part of our research. To explore the feasibility of new catalysts for the intermolecular direct addition of aldehydes/ketones to alkynes, we have developed a computational chemistry workflow whereby putative catalyst intermediates are optimized by DFT before and after carbon–carbon bond formation, and the Gibbs free energy is calculated for each, with the assumption that a working catalyst must have an exergonic C–C bond formation step. One example of a novel catalyst design that we are exploring is the heterocyclic 'half-salen/salan-like' system exemplified in Fig. 6, which shows putative catalytic intermediates and calculated relative Gibbs free energies for the addition of acetone to acetylene with several different catalysts in dichloromethane. Our studies suggest that pyrrolidine-based systems may work better than other secondary amines with Cu(I) (Fig. 6A vs 6B), and that reduced 'salan-like' catalysts are more promising than 'salen-like' Cu(I) catalysts (Fig. 6C vs 6D). Calculated Cu(I) geometries are consistent with the distorted tetrahedral geometries that have been observed in various x-ray structures.
6. Synthesis of salen- and salan-like precatalysts
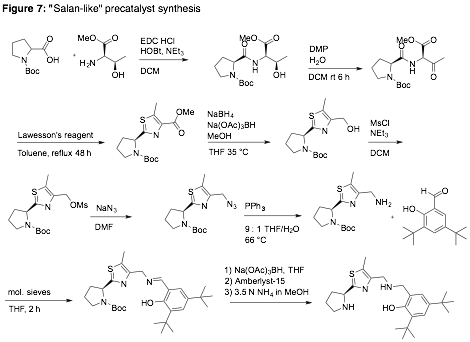
We have completed the synthesis of several salen- and salan-like precatalysts, with a representative example depicted in Fig. 7. Alkyne-binding studies with several catalysts, as well as reaction screens for the addition of aldehydes/ketones to alkynes, are in progress.
Conclusions and current/future work
We now have a library of precatalysts in hand with numerous metal chelating functionalities, and several proof-of-concept bifunctional catalysts for direct aldol reactions have been obtained. A platform for precatalyst design and preliminary evaluation has been developed using DFT calculations, which we have applied to the design of a novel class of 'salan-like' catalysts that are presently under investigation for unprecedented intermolecular additions of aldehydes/ketones to alkynes and other electrophiles.
References
[1] Wiedenhoeft, D.; Benoit, A. R.; Porter, J. D.; Wu, Y.; Virdi, R. S.; Shanaa, A.; Dockendorff, C.* Synthesis 2016, 48, 2413.
[2] Wiedenhoeft, D.; Benoit, A. R.; Wu, Y.; Porter, J. D.; Meyle, E.; Yeung, T. H. W.; Huff, R.; Lindeman, S. V.; Dockendorff, C.* Tetrahedron 2016, 72, 3905.
[3] Wang has taken a related hybrid catalyst approach to aldol reactions: Xu, Z.; Daka, P.; Budik, I.; Wang, H.; Bai, F.-Q.; Zhang, H.-X. Eur. J. Org. Chem. 2009, 27, 4581.
[4] Montaignac, B.; Praveen, C.; Vitale, M. R.; Michelet, V.; Ratovelomanana-Vidal, V. Chemical Commun. 2012, 48, 6559.
[5] Tang, X.; Kuang, J.; Ma, S. Chem. Commun. 2013, 49, 8976.
[6] Fujimoto, T.; Endo, K.; Tsuji, H.; Nakamura, M.; Nakamura, E. J. Am. Chem. Soc. 2008, 130, 4492.