Reports: UNI154284-UNI1: Alkene Acylation by Photoredox Catalysis
Patrick H. Willoughby, PhD, Ripon College
Introduction. Insertion reactions are among the most efficient processes for generating molecular complexity within a single chemical operation. We have been developing reaction conditions that facilitate the net insertion of aldehydes into electronically activated amides to give N-phthalimido-O-acyl-N,O-acetals. N,O-Acetals are a privileged class of molecules because of their role in the bioactivity of several natural products (e.g., irciniastatin A and pederin) and their ability to serve as bench stable imine surrogates. The following report will discuss our efforts into reaction optimization, mechanistic studies, and substrate scope evaluation.
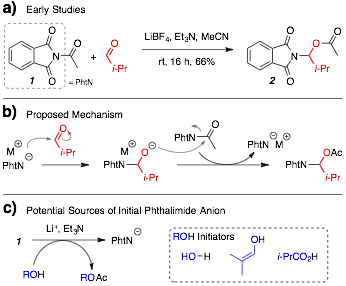
Early Efforts. We observed that treating a mixture of acylphthalimide 1 and isobutyraldehyde with LiBF4 and triethylamine led to the efficient production of N,O-acetal 2. A positive crossover experiment led us to propose a mechanism (cf. Figure 1b) where a liberated phthalimide anion engages the aldehyde to form an alkoxide, which is subsequently acylated by acylphthalimide to form the desired product and regenerate phthalimide anion. The proposed mechanism requires free phthalimide anion at the outset of the reaction, which we propose is generated by reaction between acylphthalimide and an OH donor, such as water, aldehyde enol content, and/or carboxylic acid impurities from aldehyde autoxidation (cf. Figure 1c).
Figure 1. Early Efforts and Mechanistic Proposal
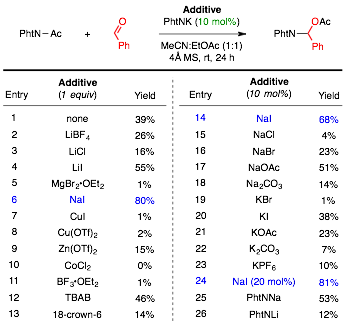
Reaction Optimization. The proposed mechanism depicted in Figure 1b suggests that phthalimide anion is a catalyst for the transformation as it is regenerated during the formation of product. With this in mind, we began our optimization studies (cf. Table 1) by treating a mixture of benzaldehyde and acetylphthalimide (PhtN–Ac) with 10 mol% of phthalimide potassium salt (cf. entry 1). Consistent with the proposed mechanism, N,O-acetal product was formed, albeit in a reduced yield of 39%. After screening a variety of stoichiometric additives (cf. entries 2–13), we found that use of sodium iodide gave the most improved yield of 80%. When compared against other sodium and potassium salts (cf. entries 14–23), NaI still gave the highest yield at 10 mol%, and product yield increased further when 20 mol% of potassium phthalimide and sodium iodide were used (cf. entry 24). Sodium phthalimide was also an efficient catalyst at 10 mol% (entry 25), and showed an improved yield compared to use of either potassium phthalimide (entry 1) or lithium phthalimide (entry 26).
Table 1. Reaction Optimization Studies
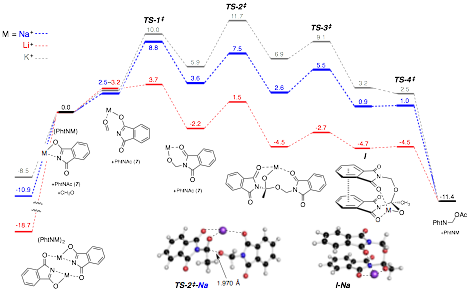
Detailed Mechanistic Studies. Computational studies were carried out to determine the feasibility of the proposed mechanism and gain insight into the role of the metal counterion in facilitating the transformation (cf. Figure 3). Using density functional theory [M06/6-31+G(d,p) with SMD solvation (MeCN)] the energies of the intermediates and transition states along the potential energy surface were computed for the formation of N,O-acetals from acylphthalimides and aldehydes with potassium (gray pathway), sodium (blue pathway), and lithium (red pathway). These studies provided strong support for our proposed mechanism and provided insight into the effect of the metal counterion.
Figure 2. Computational Studies of Reaction Mechanism
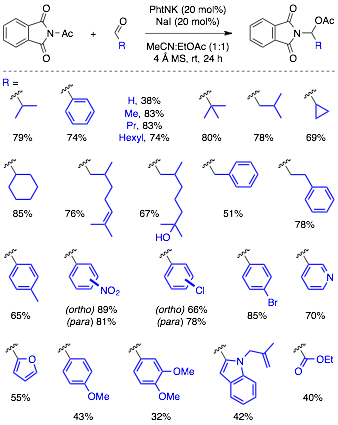
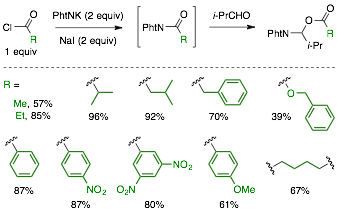
Substrate Scope of Aldehydes. The scope of aldehydes that react with acylphthalimides to form N,O-acetals was found to be quite broad (cf. Figure 3). Aliphatic aldehydes with varying steric hinderance and substitution patterns readily underwent the reaction. A variety of benzaldehydes also participated in the reaction. Electron-rich benzaldehydes gave reduced yields under the optimized conditions, which led us to increase the loading of the sodium iodide and potassium phthalimide additives to improve yields to useful levels.
Figure 3. Scope of Aldehyde Substrates
One-Pot Conditions and Substrate Scope of the Acyl Group. One-pot conditions were developed to avoid having to prepare and isolate the acylphthalimide precursor (cf. Figure 4). Specifically, N,O-acetal products were formed from potassium phthalimide, acid chloride, and aldehyde. Under these conditions, 2 equiv of NaI and potassium phthalimide were used to ensure efficient in situ generation of the acylphthalimide. The one-pot conditions were amenable to a number of acid halides, including benzyl chloroformate and adipoyl chloride.
Figure 4. Scope of Acyl Groups Under One-Pot Conditions
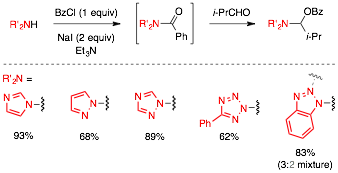
One-Pot Conditions and Substrate Scope of the Nitrogen Source. The one-pot conditions previously developed were used to probe if other heterocyclic amines would be amenable to the reaction conditions (cf. Figure 5). We found that several azoles readily participated in the reaction. It is noteworthy that 1,2,4-triazole and 5-phenyltetrazole gave product as a single regioisomer. Additionally, the crude one-pot product mixtures are sufficiently clean and rarely require additional purification, which was important because the products containing imidazole and 1,2,4-tetrazole were not amenable to normal phase column chromatography.
Figure 5. Scope of Nitrogen Source
Impact of the Research on PI Career. The results discussed in this report enabled PI Willoughby to publish the first manuscript of his independent career. In addition to the results outlined in this report, several new projects have emerged involving the synthesis of N,O-acetals. For example, we have promising results involving a stereoselective preparation of N,O-acetals from common chiral auxiliary reagents. Catalytic enantioselective methods are being developed to enable the enantioselective synthesis of N,O-acetals from achiral starting materials. Collectively, these points demonstrate that the ACS PRF UNI Grant has supported the development of a strong foundation to enable PI Willoughby the ability to provide opportunities for students to perform research in a productive setting.
Impact of the Research on Student(s) Careers. The students that participated in the research program supported by the ACS PRF UNI Grant have had the opportunity to perform research in synthetic organic chemistry and present their work at ACS National Meetings. Additionally, we recently published an article and filed a patent on our early efforts, which provided the students with a deep insight into the peer review and patent filing processes. The skills learned performing research, presenting in front of technical audiences, and submitting professional documents will certainly be useful for this promising group of aspiring scientists.