Reports: ND154823-ND1: Acid Catalyzed Alkylation Reactions of Trichloroacetimidates
John D. Chisholm, Syracuse University
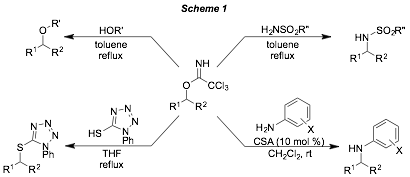
Trichloroacetimidates are known to be excellent alkylating agents when activated by a catalytic amount of a Bronsted acid. Some preliminary forays into this area showed that many imidates are much more reactive than predicted by literature studies, with alcohols, thiols and sulfonamides being alkylated by the imidates without the need for a catalyst (Scheme 1). In addition, other nucleophiles like anilines have been shown to be competent nucleophiles in the presence of an acid catalyst.
The alkylation of alcohols appears to be limited to more reactive imidates, but is still useful in the installation of 4-methoxybenzyl (PMB) and diphenylmethyl (DPM) protecting groups. The alkylation of thiols, sulfonamides, and anilines has a wider substrate scope. Indeed, thiols were shown to be excellent alkylation partners with trichloroacetimidates under thermal conditions, with benzylic, allylic and alkyl imidates all providing excellent yields of the desired sulfide products. Sulfonamides and anilines are somewhat more limited, but show good reactivity with most benzylic trichloroacetimidates. Preliminary studies have shown that these new trichloroacetimidate substitution reactions are less prone to competing elimination than methods using sulfonate-leaving groups. Studies with chiral imidates show significant racemization in these substitution reactions, so an SN1 reaction pathway is invoked with most nucleophiles. The exception is with the thiol nucleophile, which seems to be able to proceed by either an SN1 or a SN2 pathway as dictated by the electrophile. Studies are now underway to utilize these processes in more complex systems with control of regioselectivity and enantioselectivity.