Reports: DNI453861-DNI4: Understanding the Molecular Mechanism of Biological Desulfurization to Improve Sulfur Removal from Petroleum
Christina M. Payne, PhD, University of Kentucky
We have continued working toward elucidating the catalytic mechanisms of 2'-hydroxybiphenyl-2-sulfinic acid desulfinase (DszB), an enzyme responsible for cleaving the carbon-sulfur bonds of recalcitrant thiophenic compounds contained in sour crude. We made significant progress toward completion of Objectives 1 and 3. The work toward Objective 3 has been submitted for publication and is described here.
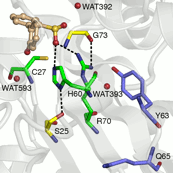
Structural and biochemical studies on DszB have been unable to resolve the reaction mechanism. It has been established that C27 is critical to activity based on inactivation upon mutation to serine. On the other hand, mutation of H60 to glutamine only reduced activity by ~17 fold. Nevertheless, H60, along with R70, is thought to be involved in the reaction, as these residues appear to hydrogen bond with HBPS in the structure (Figure 1).
Figure 1. Active site of DszB (2DE3). C27, H60, and R70 (green) have been implicated in desulfination of 2'-hydroxybiphenyl-2-sulfinate (tan). S25 and G73 (yellow), and water molecules (red spheres) hydrogen bonded to the substrate and R70 were included in the model. Y63 and Q65 (violet) are not involved in the reaction, but their mutation affects activity.
To determine the feasibility of proposed reaction mechanisms (Schemes 1 and 2) and establish the roles of active site residues, density functional theory calculations were performed using cluster models of DszB. A model consisting of HBPS and the C27 side chain (Model 1) was initially used to assess the proposed reaction mechanisms. Potential energy surface (PES) scans in the gas phase were performed at the B3LYP/6-31+G(d,p) level to locate transition states.
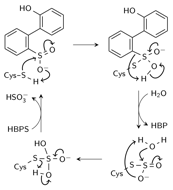
Scheme 1. Nucleophilic addition mechanism
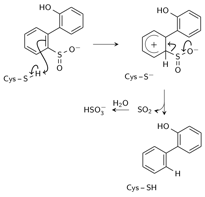
Scheme 2. Electrophilic aromatic substitution mechanism
Based on the structure, a mechanism involving nucleophilic attack on the sulfinate sulfur by C27 to break the C-S bond and form a thiosulfonate-like intermediate (Scheme 1) was proposed. The sulfinate group would serve as the general base that activates cysteine. Bisulfite is subsequently released by hydrolysis. The PES along the SCys-SHBPS reaction coordinate showed a transition state at dS-S = 2.2 Å with an energy of 99 kcal/mol. At dS-S = 2.1 Å, the hydroxyl hydrogen transferred to the sulfinate oxygen, which in turn dissociated from the sulfinate sulfur and formed a bond with the cysteine sulfur. Initial hydrogen transfer from cysteine to a sulfinate oxygen prior to nucleophilic attack was also considered. No transition state was located for hydrogen transfer to form the sulfinic acid-deprotonated cysteine intermediate.
A mechanism modeled after that of tyrosine phenol-lyase, involving electrophilic substitution of the sulfinate group by the C27 proton was also proposed. The released SO2 reacts with H2O to form HSO3- and H+ (Scheme 2). The higher reactivity of alkyl- substituted HBPS supports this mechanism; an alkyl group at the ortho or para position would stabilize the putative carbocation intermediate. A transition state was found by scanning along the HCys-CHBPS reaction coordinate, with a Gibbs free energy of activation (ΔG‡) of 31.2 kcal/mol (Table 1). The reaction is endergonic by 11.9 kcal/mol. Replacing the sulfhydryl group with hydroxyl increased ΔG‡ to 43.5 kcal/mol, consistent with inactivity of the C27S mutant.
Table 1. Thermodynamic parameters (kcal/mol) for desulfination of 2'-hydroxybiphenyl-2-sulfinate (HBPS) calculated at the B3LYP/6-31+G(d,p) level using different active site models of DszB.
a Residues are truncated at Cα and saturated with hydrogen, except G73, which includes backbone atoms. b R70 is deprotonated. c The substrate is biphenyl-2-sulfinate instead of HBPS.
The active site model was expanded to evaluate roles of surrounding residues. Addition of the H60 (Model 2) or R70 side chain (Model 3) did not lower the reaction barrier (Table 1). Inclusion of both residues (Model 4) lowered ΔG‡ by 1.7 kcal/mol (Table 1). A sulfinate oxygen is hydrogen bonded to histidine (1.8 Å) and the other to arginine (2.1 Å) at the transition state. The reaction barrier did not change upon addition of WAT392 (Model 5) hydrogen bonded to the sulfinate oxygen (Table 1). This suggests this water molecule does not play a role in stabilizing the transition state, although it is likely the one hydrolyzing the released SO2 to bisulfite.
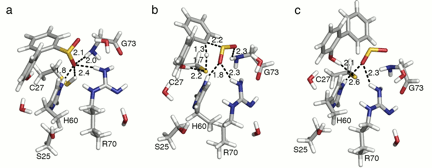
Figure 2. Structures of the (a) reactant, (b) transition state, and (c) product calculated at the B3LYP-D3/6-31+G(d,p) level using the largest model (Model 9). Bond distances are given in Å.
Model 6 includes G73, decreasing ΔG‡ by 1.3 kcal/mol (Table 1) relative to Model 5. The backbone amide hydrogen of glycine forms a hydrogen bond with the sulfinate oxygen at the transition state (2.2 Å), replacing the one with the NH2 hydrogen of arginine. ΔG‡ further decreased to 26.8 kcal/mol (Table 1) upon inclusion of S25 (Model 7). Inclusion of additional water molecules in the active site model (Models 8 and 9) did not reduce the reaction barrier.
To improve description of hydrogen bond interactions in the active site, the Model 9 reactant, transition state, and product were reoptimized using dispersion-corrected B3LYP (B3LYP-D3) (Figure 2). The B3LYP-D3 ΔH‡is similar (24.8 kcal/mol), but the favorable entropy contribution(-0.6 kcal/mol) lowered ΔG‡slightly to 24.2 kcal/mol.
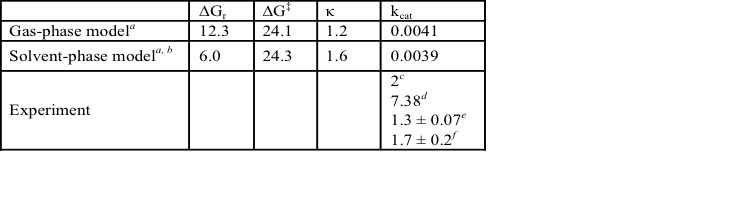
Table 2. Gibbs free energy of reaction and activation (ΔGr and ΔG‡, kcal/mol), transmission coefficient (κ), and rate constant (kcat, min-1) for DszB desulfination.
a B3LYP-D3/6-311+G(3df,2p)//6-31+G(d,p), 308.15 K. b implicit solvation using Solvation Model based on Density. c pH 7.5, 303.15 K. d pH 7.0, 301.15 K. e pH 7.4, 308.15 K. f pH 7.0, 303.15 K.
Table 2 lists the reaction free energy ΔGr, ΔG‡, and rate constant kcat obtained by single-point calculations using a larger basis set, 6-311+G(3df,2p). The calculated values, despite discrepancy with experimental data, support an electrophilic aromatic substitution mechanism for DszB desulfination of HBPS, reminiscent of protodesulfonation, which is a well-known reaction of arylsulfonic acids. H60 stabilizes the transition state through hydrogen bonding with the leaving sulfinate group. S25 modulates the charge on H60. The weaker interaction with R70 and G73 suggests these residues are less critical to transition state stabilization. The proposed electrophilic aromatic substitution mechanism is consistent with activity assays of C27S and H60Q mutants and kinetic experiments with HBPS, BPS, and alkyl-substituted HBPS.