Reports: UR1055088-UR10: Investigation of Pre-Transition Droplet Formation in Pseudobinary Liquid-Liquid Systems
J. Charles Williamson, PhD, Willamette University
This annual report encompasses the first fifteen months of grant activity, including two summers and one academic year. The broad goal of the project is to characterize the pre-transition droplet formation (PTDF) phenomenon that has been observed in pseudobinary liquid-liquid systems. Four objectives were delineated in the original proposal to meet this goal:
A. Assess the effect of extrinsic impurity doping on PTDF.
B. Explore the effect of isotopologue abundance percentages on PTDF.
C. Physically isolate pre-transition droplets and determine their composition.
D. Explore PTDF in liquid-liquid systems featuring Coulombic interactions.
Thus far, progress has been made on the first three objectives.
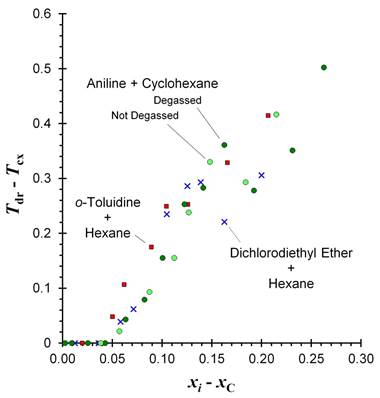
Objective A: Preparatory work for extrinsic impurity doping tests was completed by measuring the phase behavior of the aniline + cyclohexane (ACH) liquid-liquid system. Pre-transition droplet formation was observed in the ACH system, with droplet formation occurring on the aniline-rich side of the phase diagram. The quantitative behavior of PTDF was identical to PTDF behavior observed previously in six other liquid-liquid systems. Figure One shows the difference in temperature between the onset of PTDF and the bulk liquid-liquid phase transition, Tdr – Tcx, as a function of sample composition relative to the critical composition. For comparison, aniline + cyclohexane data are juxtaposed against o-toluidine + hexane data collected previously in our laboratory.
A second set of ACH samples was prepared in which each mixture was not degassed by freeze-pump-thaw prior to flame-sealing the ampule. The PTDF behavior remained unaffected by the dissolved gases (Figure One). Assessing the impact of dissolved gases on PTDF was needed for Objective C, where larger-scale ACH samples will be prepared, and it will not be possible to degas them.
Figure One: Pre-transition droplet formation behavior in the aniline + cyclohexane, o-toluidine + hexane, and dichlorodiethyl ether + hexane liquid-liquid systems. The difference between the temperature onset of droplet formation, Tdr, and the temperature of the bulk phase transition, Tcx, is plotted as a function of the mole fraction composition difference between sample and critical composition, xC. Some ACH samples were not degassed.
Objective B: Several experiments were carried out to explore the role of isotopologue variation on pre-transition droplet formation. As discussed in our proposal, the isobutyric acid + water (IAW) liquid-liquid system was the only system we had tested at that time that did not exhibit PTDF. The IAW system also has the lowest secondary isotopologue percentage (~4%). We followed up on this observation by characterizing the phenol + water liquid-liquid system, which has a secondary isotopologue percentage of about 6%. No PTDF was observed with the phenol + water system.
On the other end of the scale, we tested systems with higher isotopologue variability. In the time between grant proposal submission and acceptance, the dichlorodiethyl ether + hexane (DCEH) liquid-liquid system was characterized in our laboratory. The DCEH system was chosen because the 3:1 natural abundance ratio of 35Cl:37Cl leads to a much greater isotopologue variation in DCEH samples than in any other liquid-liquid system we have studied to date. The DCEH system showed pre-transition droplet formation on the dichlorodiethyl-ether-rich side of the phase diagram, and quantitative behavior was identical to other systems (Figure One).
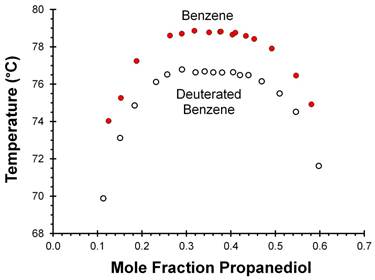
We then characterized the 1,2-propanediol + benzene (PDB) liquid-liquid system with the idea of artificially changing secondary isotopologue percentages by doping with fully-deuterated benzene. However, the PDB system did not exhibit pre-transition droplet formation, and neither did the 1,2-propanediol + deuterated benzene liquid-liquid system. The critical temperature of the deuterated system was about 2 °C lower than the PDB system (Figure Two); this difference is consistent with work published by other research groups that has shown an 0.3 °C drop in critical temperature per non-hydrogen-bonding hydrogen atom replaced by a deuterium atom.
Figure Two: Liquid-liquid phase behavior of the 1,2-propanediol + benzene and 1,2-propanediol + deuterated benzene (C6D6) systems.
Objective C: A new laser light scattering instrument was designed with the goal of extracting pre-transition droplets for further characterization. Instrument construction is now 95% complete (Figure Three). The instrument features (i) the capacity to handle 100-mL samples, as opposed to our standard 2.5-mL samples; (ii) temperature stability of ±0.01 C; (iii) a pair of lasers spaced vertically along the sample axis to assess pre-transition droplet migration; (iv) two small-angle light scattering detectors and one 90° light scattering detector; (v) a telescope for observing droplets at the upper meniscus of the sample; and (vi) a septum port in the top of the 100-mL ampule to allow the extraction of droplets with a syringe.
Figure Three: Laser light scattering instrument designed for pre-transition droplet extraction from liquid-liquid mixtures.
Impacts: Three undergraduates participated in our summer research program through the support of this grant. Two of these students presented a poster describing their work at the 24th Murdock College Science Research Conference. Two undergraduates worked on this project during the academic year, and results from this project were central to their Senior Theses. One of those students is now enrolled in a chemistry graduate program.