Reports: ND554149-ND5: Impact of Microenvironment on the Catalytic Activity of Supported Gold Nanoclusters
Jennifer Shumaker-Parry, PhD, University of Utah
The research goal has been to generate controllable microenvironments for silica-supported gold nanoclusters (AuNCs) and to investigate the impact of the microenvironment on catalysis. Our initial studies focused on large silica nanoparticles (SiNPs) as a support material for metal nanoclusters. We successfully prepared aminated SiNPs, grew small (< 5 nm) AuNPs on the surface, and introduced a polymer brush on the silica surface through atom transfer radical polymerization of 2-hydroxyethyl methacrylate. We examined the catalytic activity of these nanoparticles using a model reaction, reduction of 4-nitrophenol (4-NP) to 4 aminophenol (4-AP) with NaBH4. One challenge of the silica support system is that under the basic conditions of this model reaction, the metal nanoparticles form aggregates over time due to the dissolution of the SiNPs. This led to investigations of nanodiamond (ND) as a chemically and mechanically robust support material for metal nanoparticle catalysts. Although we continue to pursue functionalized SiNPs as supports used in catalysis reactions taking place under more mild conditions, we have also undertaken more extensive studies of the ND catalyst supports.
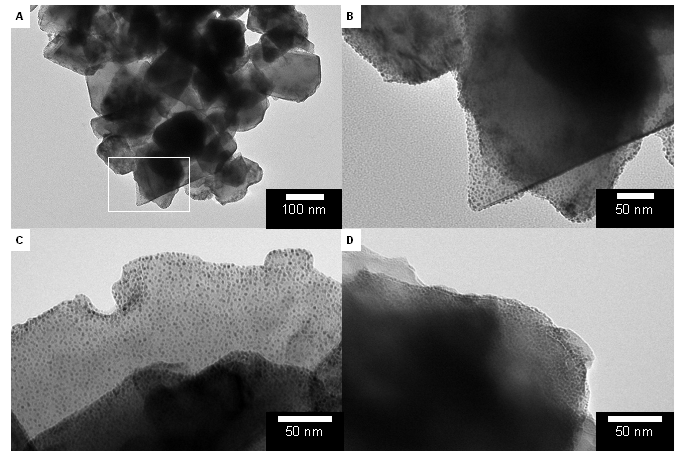
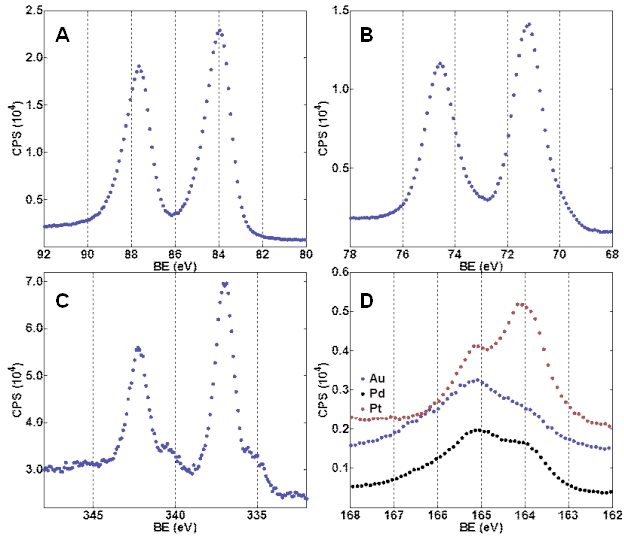
We used thiol-ene chemistry for both attachment of a polymer film to ND and subsequent immobilization of metal nanoparticles on the ND surface (Figure 1) Through high resolution scanning/transmission electron microscopy (S/TEM) analysis we observed that a mixed polymer thin film coating on the ND could be formed (Figure 2). In addition, Au, Pt, and Pd nanoparticles could be formed in situ and were found to be tethered to the surface of the polymer-coated NDs. X-ray photoelectron spectroscopy (XPS) analysis confirmed the presence of the metal NPs observed in the TEM images. The XPS spectra (Figure 3a-c) also demonstrated that the metal NPs are localized on the surface of the polymer coating because the Au 4f, Pt 4f, and Pd 3d regions are clearly observable and XPS is most sensitive to the composition in the top few nanometers of the surface. If the metal NPs were embedded inside polymer layers that are 5-29 nm thick then the metal photoelectrons would be completely blocked. The peak shapes and positions of both Au 4f7/2 (84 eV) and Pt 4f7/2 (71 eV) seem to indicate that most of these atoms are in the Au(0) and Pt(0) state. However, the location and peak shape of Pd 3d5/2 indicates that the PdNPs/polymer/ND material is mostly decorated with Pd(II) (337 eV) and that Pd(0) (335 eV) is much less abundant. This difference between the abundance of oxidation states for Au and Pt vs Pd is likely a result of several factors: oxide formation, Pd-S bonding, and incomplete reduction of the precursor Pd salt. TEM images unambiguously confirm the presence of PdNPs, however Pd is also easily oxidized and this oxidation may have contributed to an increase in the relative abundance of Pd(II) vs Pd(0) between the preparation of the PdNPs/polymer/ND material and subsequent XPS analysis. The presence of the polymer on the ND surface was confirmed by S 2p peaks in the XPS spectra (Figure 3d). The lower intensity of the peaks observed for sulfur compared to that of metal NPs is an indication that the polymer adhesion layer is likely a significantly smaller fraction of the total mass of the composite particles.
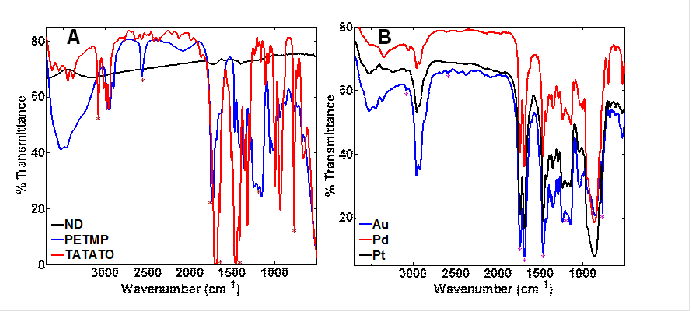
High resolution energy dispersive spectroscopy (EDS) obtained with scanning TEM (S/TEM) provided nanoscale chemical mapping of the polymer and Au, Pt, or Pd NPs attached to the polymer/ND surface. We also used diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) to characterize the surface chemistry environment of the metal nanoparticles (Figure 4). The spectrum for the bare NDs is smooth and featureless as expected, while the principle monomers have several identifying features above 1500 cm-1 in addition to those in the fingerprint region (Figure 4a), such as carbonyl peaks characteristic of the PETMP and TATATO monomers at 1738 cm-1 and 1688 cm-1, respectively. These same carbonyl peaks are observed in the composite particles (Figure 3b) indicating that the PETMP and TATATO were incorporated into the polymer. Furthermore, because the molar ratio of PETMP:TATATO is 3:4, the carbonyl peaks in the DRIFTS spectra of polymer/NDs have similar intensities. Other characteristic peaks are located at 3085 cm-1 (weak), 1466 cm-1 (strong, sharp), 765 cm-1 (sharp) in the IR spectra of the composite particles and in the IR spectrum of pure PETMP. Three peaks centered at 1185 cm-1 are present in the composite particles as well as the IR spectrum of pure TATATO.
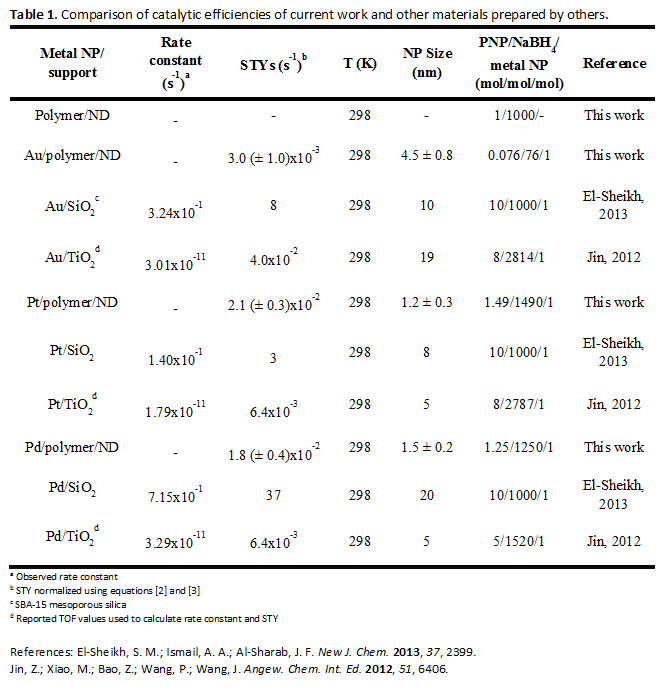
When used as the catalyst for the reduction of PNP, the site time yields (STYs) for Au, Pt and Pd NPs immobilized on polymer/NDs were within a typical range of reported values for Au and Pd NPs, with AuNPs being less catalytically active than Pd (Table 1). However, the activity of our PtNPs is higher than expected relative to the Au and Pd NPs. The attachment of the PtNPs to the polymer may influence the adsorption energy of PNP leading to a more favorable reaction environment than that of unsupported PtNPs. As a comparison, STYs for 4-NP reduction for catalyst NPs supported on SiO2 and TiO2 are presented in Table 1. In these examples, SiO2 supported NPs exhibit higher rates of 4-NP conversion. Although the polymer/ND composites presented in our study exhibit lower conversion rates, their stability surpasses that of SiO2 based systems.
Figure 1. Photoinitiator and monomers used in polymer coating of NDs. The latter were then decorated with metal nanoparticles via in situ reduction of metal salts.
Figure 2. TEM images of polymer/diamond supports decorated with three different types of metal NPs. (A) AuNPs/polymer/NDs. (B) Higher magnification of AuNPs/polymer/NDs. (C) PtNP/polymer/NDs. (D) PdNP/polymer/NDs.
Figure 3. XPS spectra of polymer/ND materials. (A) Au 4f region of AuNP/polymer/NDs, (B) Pt 4f region of PtNP/polymer/NDs, (C) Pd 3d region of PdNP/polymer/NDs, (D) S 2p region for all three materials.
Figure 4. Vibrational spectra of (A) monomers (neat films on NaCl) and diamond (dried powder diluted with KBr) probed by DRIFTS, (B) DRIFTS spectra for polymer/NDs with imbedded Au, Pd, or Pt NPs. Asterisks indicate features similar between monomers and polymer/NDs.