Reports: ND555692-ND5: Catalytic Pathways for Heteroatom Removal from Heavy Fossil Fuels
Michael Trenary, University of Illinois (Chicago)
1. Background
The use of heavy fossil fuels for the petroleum used transportation is increasing. A challenge to using such resources is that they contain higher concentrations of sulfur-containing compounds. Basic research is therefore needed on the reaction pathways followed in catalytic processes used for sulfur removal. In our research, we are using use surface science methods to gain new fundamental insights into mechanisms related to oxidative desulfurization (ODS) of dibenzothiophene (DBT) over a V2O5 model catalyst.
Dibenzothiophene was chosen for our initial studies because it contains a S atom in a position that makes its removal by typical hydrodesulfurization (HDS) catalysts particularly difficult. In general, it is found that dibenzothiophenes are the dominant sulfur-containing species that remain in fuels after they are subjected to the normal HDS process. In ODS, the organosulfur compounds are generally converted to polar sulfoxides or sulfones, which can be extracted from the pure hydrocarbons. To skip the expensive extraction step, it would be desirable to produce gaseous sulfur compounds. It is thermodynamically feasible to oxidize DBT to SO2 and hydrocarbons at typical refinery temperatures. However, to make this route sufficiently efficient, new catalysts with high selectivity for sulfide oxidation must be identified. For this reason, DBT and related molecules have been the subject of numerous heterogeneous catalyst studies. In contrast, powerful surface science methods of the sort being performed in this project are generally lacking and yet such studies can provide important basic information on the catalytic surface chemistry associated with removal of the S atoms from these molecules.
2. Results
2.1. Preparation and Characterization of V2O5 films on Pd(111)
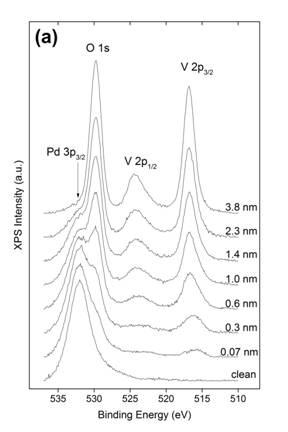
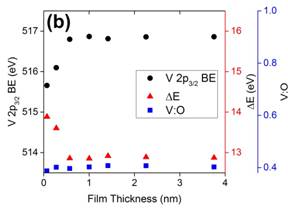
There have been numerous previous studies of the growth of vanadium oxide films on metal surfaces. These are most commonly prepared by evaporating V metal in an ambient of O2. While this method works well for generation of the oxides of vanadium in lower oxidation states, it is more difficult to produce V2O5 without extensive post-deposition oxidation. We have successfully implemented an alternative method in which we directly evaporated V2O5 from a small Knudsen cell. The films generated in this way have been examined with X-ray photoelectron spectroscopy (XPS), low energy electron diffraction (LEED), and reflection absorption infrared spectroscopy (RAIRS). The clean surface in Fig. 1(a) shows only the Pd 3p3/2 peak at about 532 eV, but as more V2O5 is deposited, this peak is diminished and the O 1s, V 2P3/2, and V 2p3/2 peaks develop. From the degree of attenuation of the Pd peak, values of the film thickness are determined as displayed in Fig. 1 (a). The V binding energies and O:V ratio confirms that this method produces a stoichiometric V2O5 thin film. Fig. 1 (b) demonstrates that although thinner films have the V2O5 stoichiometry, the influence of the Pd substrate shifts the V binding energies away from the values found in bulk V2O5.
The stability of the V2O5 films as a function of temperature was also studied. There is a loss of oxygen and a shift of the V binding energies for temperatures above 620 K. After heating to 1000 K, there is very little vanadium oxide left on the surface, but what remains has V binding energies characteristic of the V+3 oxidation state. The RAIRS results reveal a very sharp peak at 1041 cm-1 characteristic of a V=O double bond stretch. The spectra undergo changes as the initially deposited amorphous film is converted to a more crystalline form. This transformation is manifested by observation of a variety of ordered structures with LEED. After annealing to 620 K, 2×2 spots appear in the LEED pattern, which persists up to 680 K. Annealing to higher temperatures leads to clusters of spots around the 1×1 spots, indicating formation of a structure with a large lattice constant. In the RAIR spectra, the V=O stretch reaches its minimum width of 4.1 cm-1 after annealing to 620 K, indicating that this is the temperature of maximum crystalline order in the V2O5 film. The results demonstrate that a stoichiometric, ordered V2O5 film can be formed on Pd(111) and that it is stable up to at least 600 K. This then implies that surface chemical reactions, such as ODS, over V2O5 films can be carried out over a wide temperature range. A publication on the deposition and characterization of V2O5 on Pd(111) is nearly ready for submission.
2.2 Interactions of DBT with V2O5/Pd(111)
We have used RAIRS, XPS, and temperature programmed desorption (TPD) to study the adsorption and reactions of DBT with the V2O5 film. RAIRS indicates that the molecule adsorbs parallel to the surface as only the out-of-plane C-H bending vibrations are observed. As the surface is heated, the DBT appears to mainly desorb molecularly. No oxidation products are observed to desorb with TPD, nor are any vibrational features attributable to oxidation products observed with RAIRS. However, XPS does reveal shifts in the V peaks that may indicate vanadium reduction, a reaction expected to accompany DBT oxidation. Experiments to find other conditions under which DBT oxidation occurs more readily are currently underway.
2.3 Impact on Careers of PI and Students
This grant is allowing me, the PI, to explore a new area of research. I hope to use the results obtained in other proposals to continue work in this area. The funding has been used to support two graduate students, and to partially support a postdoctoral researcher. One of the students has successfully completed his PhD degree and is currently employed as a research chemist in industry. The other student is benefiting from PRF support as he pursues his PhD research.
Figure 1. (a) Pd 3p3/2, O 1s and V 2p XP spectra of V2O5 on Pd(111) at various coverages. (b) Plots of V 2p3/2 binding energy, difference in binding energy (ΔE) between O 1s and V 2p3/2, and V:O ratio as a function of V2O5 film thickness. All XPS spectra were acquired at 50 eV pass energy using Mg Kα radiation for the substrate temperature at 300 K.