Reports: DNI1055526-DNI10: Effects of the Chemistry and Structure of Petrochemical Polymers on the Vapor-Phase Conversion to Organic-Inorganic Hybrid Materials
Mark D. Losego, Ph.D., Georgia Institute of Technology
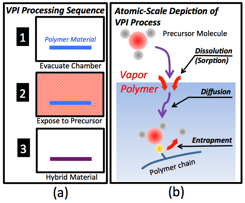
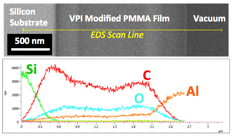
During the first year of this ACS PRF DNI Award funding, the PI's research team has pioneered a new processing technique—vapor phase infiltration (VPI)—for enhancing the properties of commodity petrochemical polymers. VPI is a chemical vapor processing method to transform polymers into novel organic-inorganic hybrid materials with unique properties. VPI exposes polymers of various form factors (e.g., bulk, fibrous, film) to gaseous metalorganic precursors (Fig. 1a). These gaseous species dissolve and diffuse into the polymer and subsequently become entrapped within the matrix either through chemical reaction or steric impediment (Fig. 1b). Fig. 2 shows unpublished data from the Losego Lab demonstrating the ability for VPI to infiltrate inorganic constituents into the bulk of a polymeric material—in this example aluminum is diffused more than 2 microns into the depth of a PMMA film.
Fig. 1: (a) Schematic of VPI process. (b) Depiction of the atomic scale processes important in VPI.
Fig. 2: Unpublished SEM data from the Losego Lab showing an EDS line scan profile of aluminum infiltration into a PMMA film via VPI processing.
During this project period, the Losego research team has (1) used VPI to enhance the properties of several common petrochemical polymers, (2) developed new methods to assess the chemical and structural features of these materials, and (3) worked to derive fundamental transport equations to theoretically describe the VPI processing kinetics. Fig. 3 illustrates one of the PI's recent discoveries of VPI materials with enhanced properties. Here, a thermoplastic polymer exposed to VPI is made resistant to dissolution in organic solvents. To the best of our knowledge, these VPI processes are modifying BOTH the surface and sub-surface chemistry of the polymer, making these treatments more robust than their purely surface treatment counterparts.
Fig. 3: Example of a VPI treated polymers with enhanced properties: a thermoplastic that does not dissolve in organic solvents.
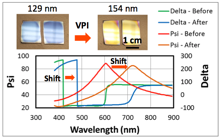
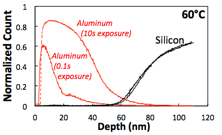
The research team has also successfully developed several protocols for characterizing the structure and chemistry of the novel organic-inorganic hybrid materials produced with VPI. These methods include spectroscopic ellipsometry, secondary ion mass spectrometry (SIMS), cross-sectional scanning electron microscopy (SEM) with energy dispersive spectroscopy (EDS) compositional mapping, and Fourier transform infrared spectroscopy (FTIR). Fig. 4 shows example ellipsometry data before and after VPI on a PMMA thin film. Shifts in ellipsometric functions are readily apparent and can be used to discriminate between and track the changes in film thickness and optical constants (e.g., refractive index). These values can then be correlated to the infiltration fraction. As presented in Fig. 5, SIMS can be used to more directly probe the concentration gradient of a VPI modified material. Here, aluminum and silicon (substrate) concentration profiles are shown to demonstrate the effect of exposure time on TMA VPI of PMMA. By fitting these compositional profiles to analytically derived diffusion-reaction models, this data can be used to understand the VPI processing kinetics.
Fig. 4: Example ellipsometric data collected from a PMMA film before and after VPI. Top inset shows photos of PMMA films before and after VPI treatment. (Losego, Unpublished)
Fig. 5: Representative SIMS data of an aluminum VPI diffusion profile in PMMA. (Losego, unpublished)
The Losego research team is also working to derive phenomenological models for the infiltration kinetics of the VPI process. These types of models are essential for predictively designing VPI process parameters. VPI kinetics involves a competition between precursor diffusion and precursor reaction. To better elucidate these mechanisms, the PI's research team has begun to derive analytical models based on Fick's 2nd Law for the relevant diffusion conditions in VPI under various geometric constraints (e.g., film on impermeable substrate and cylindrical fibers). Competing reaction rates have been added to these equations using various assumptions (e.g., instantaneous reactions that simplify the result to a moving boundary problem). By comparing the functional forms of these equations to measured compositional profiles, our research team is attempting to clarify the VPI processing mechanisms.
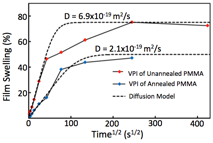
Fig. 6 demonstrates the ability to fit our measured data to these models. Here ellipsometry data collected for film swelling at various VPI processing times is fit to a purely diffusional model. By plotting film swelling versus the square root of time, the diffusion coefficient can be extracted from the slope of the initial linear portion of the data. In some cases, a deviation from the model occurs, and we are currently working to understand how this deviation relates to either the onset of reaction or a change in the diffusion constant.
Fig. 6: Example of ellipsometry data fit to our VPI processing kinetics model. (unpublished)
Generous support from this ACS PRF Doctoral New Investigator Award has benefited both the PI's career trajectory and his students' educational progress. Over the past year, results from this work have initiated new interdisciplinary research endeavors between the PI and his colleagues at Georgia Tech working in such diverse fields as gas membrane separations and anti-bacterial surfaces. Preliminary results from this research program have also helped the PI secure new external funding from two separate industrial sponsors. The graduate student supported on this project has successfully passed his Ph.D. qualifying exam, presented a poster about his research at an American Ceramics Society conference, and been awarded the Thin Film Division Graduate Student Award from the American Vacuum Society. An undergraduate researcher supported on this project has been afforded the opportunity to gain valuable skills in controlled free radical polymerization in support of his career goal of securing a job in the polymer industry (see Fig. 7). This undergraduate researcher has also presented his work in poster form at three separate Georgia Tech symposia.
Fig. 7: Undergraduate researcher performing an air-free controlled free-radical polymerization experiment as part of this ACS PRF DNI Award.