Reports: DNI1054898-DNI10: Corrosion in Extreme Environments
Hojong Kim, PhD, Pennsylvania State University
Introduction.
Exploration and extraction of oil and gas wells require the use of corrosion-resistant materials that can withstand harsh chemical surroundings containing hydrogen sulfide (H2S), carbon dioxide (CO2), and brines (e.g., alkali/alkaline-earth halides and sulfates) under high temperature and pressure conditions. In H2S-containing sour environments, engineering materials (e.g., steels) are susceptible to abrupt mechanical failures, known as sulfide stress cracking where hydrogen atoms as a product of the corrosion process diffuse into the base metal and embrittle the crystalline structure. In sulfide stress cracking, sulfide films (e.g., FeS2) are also present at the metal/environment interface as corrosion products. However, the mechanistic role of these film in interfacial corrosion processes (e.g., hydrogen reduction reactions) is not fully understood, possibly due to their complex phase structures. Enabled by this ACS PRF grant, this work investigates the interfacial electrochemical properties of sulfide films (e.g., pyrite, FeS2) in aqueous solution to elucidate its chemical interactions with H2S-containing aqueous environments. This study specifically aims to understand the hydrogen reduction kinetics at the pyrite surface and eventually the mass transport kinetics of hydrogen atom through the pyrite film.
Methods and Results.
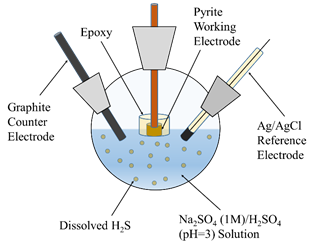
In the last year, the hydrogen-absorption behavior of the pyrite crystals was studied using an electrochemical cell in Na2SO4-H2SO4 solution at pH 3 under varying concentration of H2S (10-30 ppm), as shown in Figure 1. The dissolved H2S was introduced into the solution phase by dissolving the controlled quantities of sodium sulfide (Na2S) in the acidic solution of Na2SO4 (Na2S + H2SO4 → H2S + 2Na2SO4). A suite of electrochemical techniques was employed, including cyclic voltammetry, linear polarization, and electrochemical impedance spectroscopy (EIS) techniques.
Figure 1. Schematic of electrochemical cell used to investigate the electrochemical properties of pyrite in H2S-containing aqueous solution.
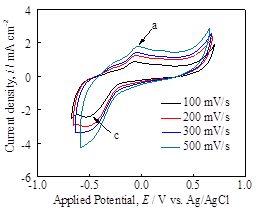
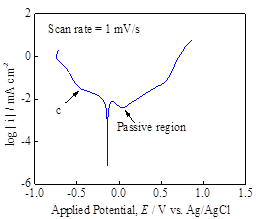
In order to understand the characteristic reduction potential of H+ ion on the pyrite surface, the pyrite working electrode was polarized in both positive and negative directions to obtain cyclic voltammograms at scan rates of 100-500 mV/s (Figure 2a). A reduction wave related to the reduction of hydrogen ion (H+) was observed, starting at -0.2 V vs Ag/AgCl. The native pyrite film usually forms oxysulfide surface layer (FeS2Ox) in the presence of air or aqueous environment, and thus, the reduced hydrogen atom is thought to be incorporated into pyrite lattice according to: FeS2Ox (lattice) + H+ + e = FeS2OxH (lattice). In addition, polarization curve was obtained using linear polarization technique at the slow scan rate of 1 mV/s (Figure 2b). The passivation characteristics of the pyrite film was observed during the anodic polarization while the hydrogen reduction reactions were observed during the cathodic polarization. These measurements serve as baseline to contrast the effects of H2S in the aqueous environment.
Figure 2. (a) Cyclic voltammograms obtained using pyrite electrode at 100-500 mV/s, depicting the H+ reduction wave and (b) linear polarization plot at 1mV/s, indicating the passivation characteristics of pyrite in anodic sweep.
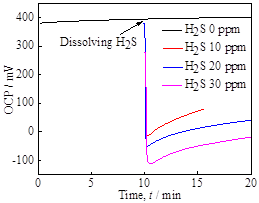
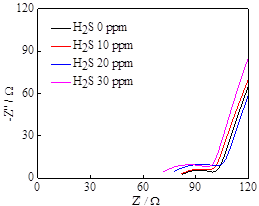
The electrochemical properties of pyrite film were investigated under the systematic control of dissolved H2S concentrations. The dissolved H2S is a weak acid and may decrease the pH of the solution by dissociation reaction (e.g., H2S = HS- + H+). Upon the introduction of H2S, the open-circuit potential (OCP) of the pyrite working electrode changed abruptly in the negative direction, and the OCP change was more pronounced as H2S concentration increases (Figure 3a). In addition, the electrochemical impedance spectra under varying concentrations of H2S, indicate that the charge transfer resistance (semi-circle width) tends to increase at higher H2S concentration (Figure 3b), suggesting that the presence of H2S may decrease hydrogen reduction kinetics by modifying the conditions at the electrode surface.
Figure 3. (a) OCP measurements as a function of time and (b) electrochemical impedance spectra, both at varying concentrations of dissolved H2S in Na2SO4-H2SO4 solution.
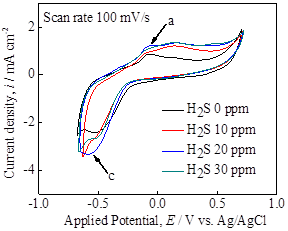
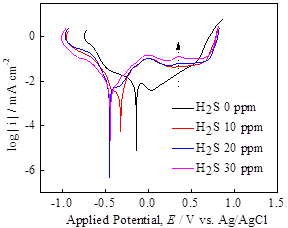
Cyclic voltammograms at various H2S concentrations exhibited a slight increase in current density at higher H2S concentration, however, the obtained reduction waves were essentially similar each other independent of H2S concentration (Figure 4a). This observation may occur due to the insignificant change in H+ concentration due to the low H2S concentration and its low dissociation constant as a weak acid. In contrast, the polarization curve in Figure 4b, shows a significant effect of H2S, in particular, the OCPs as well as the passivation characteristics during anodic polarization. It is observed that the presence of H2S in the solution phase increased the anodic current density by 1-2 orders of magnitude and widened a potential range of active dissolution. Based upon these electrochemical measurements, the stability of pyrite film seems to decrease in H2S-containing solutions. The further investigation into pyrite crystals is underway to characterize the surface structure and chemistry by scanning electron microscopy (SEM), X-ray diffractometer (XRD) and X-ray photoelectron spectroscopy (XPS).
Figure 4. (a) Cyclic voltammograms at 100 mV/s and (b) linear polarization plot at 1 mV/s, using pyrite electrode at various H2S concentrations in Na2SO4-H2SO4 solution.
Impacts of Research.
This work will establish the fundamental chemical interactions between sulfide layer with the sour environments, which will serve as a basis for better control of degradation reactions of engineering materials as well as for the design of corrosion-resistant coatings for sour oil and gas environments. Based on the achieved knowledge, the research will progress to understand the mass transport properties of hydrogen atoms through the sulfide film, using Devanathan–Stachurski electrochemical cell.
The ACS-PRF grant enabled the PI to initiate fundamental corrosion research in sour environments of metal sulfides and sulfates, to build laboratory infrastructure, and to educate and train both the undergraduate and graduate students. Electrochemical investigation of materials coupled with the chemical/structural characterization will enrich the students’ knowledge for their professional career development as scientists and engineers. Under this work, two undergraduate students completed their senior theses and a graduate student is pursuing doctoral research with the financial support from this grant.