Reports: ND552696-ND5: Two-Dimensional Nanoporous Self-Assembled Molecular Layers: New Structures for Selective Adsorption and Catalysis
Carlos Wexler, University of Missouri, Columbia
The emergence of nanoscience and the great successes in building nanotechnology have pushed the boundaries for exceptional control on the molecular and atomic scales. The ability to manipulate molecular degrees of freedom, for example, has led to the assembly of nanometer-scale materials such as carbon nanotubes [1], and zeolite-like porous materials [2]. Spontaneous molecular self-assembly is a promising route for bottom-up manufacturing of two-dimensional nanostructures with specific topologies [3,4,5,6]. Of particular interest is the possibility of selective lock-and-key interaction of guest molecules inside cavities, or pores, formed by complex self-assembled host structures. This type of surface nanostructure exhibits promising properties, such as selective adsorption and catalysis in solution. The self-assembly process is also an appealing route for bottom-up fabrication, being more efficient and cheaper than conventional top-down fabrication techniques, as the processes and mechanisms exploited are inherent to the molecular system [7]. The use of organic molecules in developing technology is also becoming an increasingly attractive route in fabrication and widespread use, for example, in organic photovoltaics and printable devices [8,9].
Our host structure is a monolayer consisting of interdigitated 1,3,5-tristyrylbenzene substituted by alkoxy peripheral chains containing n = 6, 8, 10, 12, or 14 carbon atoms (TSB3,5-Cn) deposited on a highly ordered pyrolytic graphite (HOPG) surface. The geometry of the TSB molecule, with its peripheral alkoxy chains, drives the self-assembly process through a lock-and-key mechanism, as it relaxes onto the HOPG substrate. Experimental studies have been carried out on a variety of molecules with triangular geometries, which tend to form honeycomb, or hexagonal, supramolecular networks through self-assembly [10,11,12].
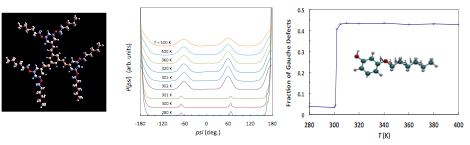
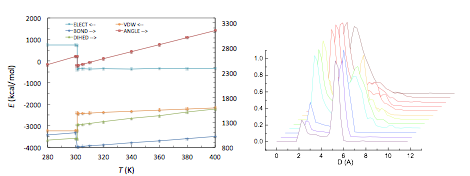
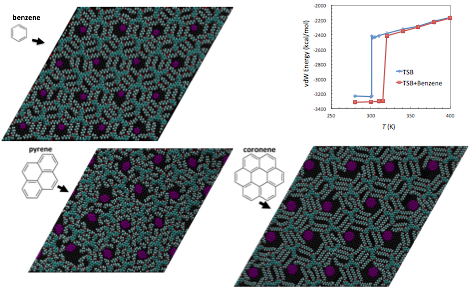
In the initial period of this project we performed ab initio quantum chemistry calculations using Gaussian 09 [13] to determine the molecular structure in vacuo and the parameters required for specifying the force field (elastic constants, charges, van der Waals constants), Fig. 1 (left). We then optimized the commensurable structure of TSB3,5-C6, and determining the equilibrium ordered configurations at 300 K. We further observed sharp temperature-driven order-disorder transitions that result in the destruction of the ordered superlattice. We further characterized the state of each phase in terms of probability distributions, Fig. 1 (center, right), and internal energies, Fig. 2 (left). We also observed and characterized the changes in the pore size distribution of the superlattice with temperature, Fig. 2 (right). In terms of the application of the TSB superlattices for selective adsorption, we observed significant stabilization of the structure in the presence of benzene and pyrene guest molecules, Fig. 3, and we also extended the results to larger guest molecules in the larger superlattice of TSB3,5-C10, Fig. 3.
Figure 1. Left: ab initio determination of the structure and partial charges of TSB3,5-C6. Center: probability distribution of of dihedral angles for various temperatures. Right: fraction of gauche defects vs. temperature (inset: depiction of dihedral angles).
Figure 2. Left: internal energies of a 4x4 superlattice of TSB3,5-C6 vs. temperature. Right: pore size distribution for various temperatures, showing the transition between stable large pores to unstable smaller pores and eventual cration of large disordered structure.
Figure 3. Left: adsorption of benzene and pyrene in a TSB3,5-C6 superlattice. Right top: melting of the superlattice (and ejection of guest molecules). Right botton: coronene on TSB3,5-C10.
REFERENCES
1. Majumder, M., Chopra, N., Andrews, R. & Hinds, B. J. Nanoscale hydrodynamics: enhanced flow in carbon nanotubes. Nature 438, 44 (2005).
2. FŽrey, G., Mellot-Draznieks, C., Serre, C., Millange, F., Dutour, J., SurblŽ, S. & Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 309, 2040–2042 (2005).
3. Barth, J. V. Molecular architectonic on metal surfaces. Annu Rev Phys Chem 58, 375–407 (2007).
4. Theobald, J. A., Oxtoby, N.S., Philips, M.A., Champness, N.R. & Beton, P.H. Controlling molecular deposition and layer structure with supramolecular surface assemblies. Nature 424, 1029–1031 (2003).
5. Griessl, S. J. H., Lackinger, M., Jamitzky, F., Markert, T., Hietschold, M. & Heckl, W.M. Incorporation and Manipulation of Coronene in an Organic Template Structure. Langmuir 20, 9403–9407 (2004).
6. Stepanow, S., Lingenfelder, M., Dmitriev, A., Spillmann, H., Delvigne, E., Lin, N., Deng, X., Cai, C., Barth, J.V. & Kern, K. Steering molecular organization and host–guest interactions using two-dimensional nanoporous coordination systems. Nat Mater 3, 229–233 (2004).
7. Barth, J. V., Costantini, G. & Kern, K. Engineering atomic and molecular nanostructures at surfaces. Nature 437, 671–679 (2005).
8. Schmidt-Mende, L., Fechtenkštter, A., MŸller, K., Moons, E., Friend, R.H. & MacKenzie, J.D. Self-Organized Discotic Liquid Crystals for High-Efficiency Organic Photovoltaics. Science 293, 1119–1122 (2001).
9. Subramanian, V., Chang, P.C., Lee, J.B., Molesa, S.E. & Volkman, S.K. Printed organic transistors for ultra-low-cost RFID applications. IEEE Transactions on Components and Packaging Technologies 28, 742–747 (2005).
10. Balandina, T., Tahara, K., SŠndig, N., Blunt, M.O., Adisoejoso, J., Lei, S., Zerbetto, F., Tobe, Y., De Feyet, S. Role of Substrate in Directing the Self-Assembly of Multicomponent Supramolecular Networks at the Liquid-Solid Interface. ACS Nano 6, 8381-8389 (2012).
11. Szabelski, P., Rzysko, W., Panczyk, T., Ghijsens, E., Tahara, K., Tobe, Y., De Feyter, S. Self-assembly of molecular tripods in two dimensions: structure and thermodynamics from computer simulations. RSC Adv. 3, 25159-25165 (2013).
12. Ghijsens, E., Adisoejoso, J., Van Gorp, H., Destoop, I., Noguchi, A., Ivasenko, O., Tahara, K., Van der Auweraer, M., Tobe, Y., De Feyter, S. On the stability of surface-confined nanoporous molecular networks. J. Chem. Phys. 142, 101932 (2015).
13. Frisch, M.J., et al., Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford CT, 2009.