Reports: UNI155657-UNI1: Tunable Triarylmethyl Carbocation Catalysis
Cheyenne S. Brindle, PhD, Trinity College
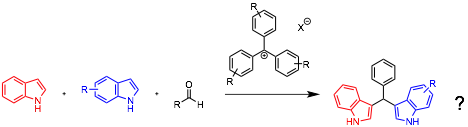
In the first year of my Petroleum Research Foundation Grant I was able to take a year-long sabbatical to dedicate my time to my research that was funded by the grant. The first pursuit was to identify reactions that could be catalyzed by triarylmethyl cations and that would illustrate the benefit of tunability, either sterically or electronically. To this end I screened many nucleophile/electrophile combinations, using trityl tetrafluoroborate as the triarylmethyl cation catalyst. This led me to identify indole as a suitable nucleophile and benzaldehyde as a suitable electrophile. The product of this reaction is actually a double addition: two indoles act as nucleophiles to produce a bisindoylmethane product. These are interesting products with a variety of biological activities, including antibiotic properties. We had previously been studying these structures, and had been using an alternate synthesis strategy. However, the previous synthetic approach, using silica gel as a catalyst, gave only moderate to poor yields.
At this point in the project a new research student, Nicholas Boekell, joined my team and began optimizing the reaction conditions for the construction of bisindoylmethanes. He found that by tuning the triarylmethyl cation stability he was able to achieve a suitable reaction rate enhancement without excessive reactivity. Sufficient reaction rate enhancement was found with the relatively stable commercially available triarylmethane dye malachite green. While less stabilized dyes react faster, the benefit of using a commercially available catalyst was deemed to be more beneficial to adoption of this new methodology in comparison with reduced reaction times. The solvent that was initially used, dicholoromethane, could be replaced with more environmentally friendly ethanol, at elevated temperatures. Because we wanted to explore more reactive triarylmethyl cations, however, we continued to employ dichloromethane as our standard conditions, as unwanted solvent interactions with the cationic center would be avoided. Nick was joined over the summer on this project by Dana Cerone, and together they examined the substrate scope. Nick focused on the aldehyde component, while Dana investigated the indole component. She found that several of the products were too fragile to isolate using silica gel chromatography, instead requiring recrystallization. This instability highlights the mildness of our reaction conditions and the benefit of catalyst tuning. By using a weakly activating catalyst we are able to access structures that are unstable to harsher conditions. Currently, we are completing our reaction optimization and our substrate scope and will publish this work soon.
While Nick took over the bisindoylmethane synthesis, I began exploring whether it was possible to get a single addition product, rather than the double addition product. By changing the aldehyde to the less reactive imine, I was able to observe the single addition product in addition to the bisindoylmethane. By tuning the imine substituent, I was able to increase this ratio to approximately 20:1 selectivity. Further reaction optimization led to a robust reaction. I investigated the substrate scope of this single addition process and found it to be excellent for electron-neutral and electron-poor substrates. Electron-rich substrates were less selective and poorly reactive, and gave only moderate yields. At this point I was joined by a new research student, Calvin Chen, who began optimizing a 2-step one pot reaction in which a second nucleophilic indole was added after the completion of the first addition to give a bisindoylmethane with two different indole components. This work is not yet complete, as the yields are only moderate at this point. In part this was due to decomposition of the starting imines due to high humidity over the summer months. I have requested a dry box from departmental funds, so I hope to address this issue soon. Once we can complete the 2-pot method, I will publish this work. The preliminary results of this research, and the double addition methodology were presented at the August meeting of the American Chemical Society in Philadelphia.
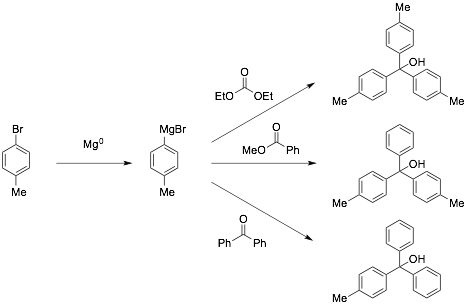
Another aspect of this research project is the synthesis of a library of triarylmethyl cation precursors. This was undertaken both by myself and Jordan Reid, a student in my research group. Jordan completed the synthesis of more than 15 different precatalysts by using a split and pool strategy that consists of Grignard formation, followed by reaction with either benzophenone, methyl benzoate, or diethyl carbonate. This synthetic strategy was highly successful in creating a diverse library of precatalysts that varied electronically and sterically. Standard Grignard formation conditions were used in most cases, but certain reagents were unstable to refluxing temperatures. For these reactions we instead employed a low temperature lithium-halogen exchange with n-butyllithium, which worked very well. This gave Jordan experience with air and water sensitive reagents that require more extensive safety precautions. It also introduced Jordan and his classmates (when he presented his research in our department seminar series) to a new reaction: the lithium-halogen exchange. This gave us the opportunity to talk about the different mechanisms that are possible for this type of process and the concept of diffusion limited reaction rates. Jordan also learned about more complex NMR concepts than he was exposed to in Elementary Organic Chemistry. Some of Jordan's precatalysts contain a fluorine atom, capable of causing splitting in the 13C and 1H NMR. In addition, we had several cases of magnetic non-equivalence, which introduced yet another dimension to the analysis. This was a great learning experience that led Jordan to explore more advanced topics in the context of his research. Currently we have over 20 different precatalysts in our library covering a wide range of activation strengths and steric hindrance.
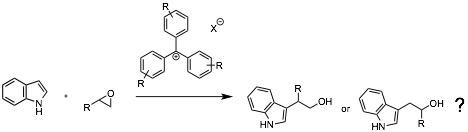
Jordan also began investigating the reaction of epoxides with indole nucleophiles catalyzed by triarylmethyl cation catalysts. This work is still in the preliminary stages. Thus far we have found that the catalysts we have attempted thus far react with indole to quench the catalyst. We plan to investigate less reactive catalysts.
In summary, we have made a lot of progress on the bisindoylmethane project and are roughly halfway through our single addition project. We have created a library of precatalysts and are exploring new reactions for further development.