Reports: DNI355281-DNI3: Metal-Organic Framework Supported Pincer Complexes: Investigation of the Effects of Site Isolation and Secondary Environment
Casey R. Wade, Brandeis University
Overview
Our laboratory has been investigating the immobilization of late transition metal diphosphine pincer complexes as linkers in metal-organic frameworks (MOFs) in order to gain insight into the effects of site isolation on reactivity and catalytic activity. Over the last year, we have developed synthetic routes to POCOP, PCCCP, and PNNNP pincer complexes decorated with carboxylic acid groups and incorporated these complexes as linkers in Zr and Cu MOFs. Initial catalytic studies with a Zr MOF constructed from Pd-POCOP metallolinkers revealed that immobilization dramatically improves catalytic transfer hydrogenation activity versus the homogeneous pincer complex.
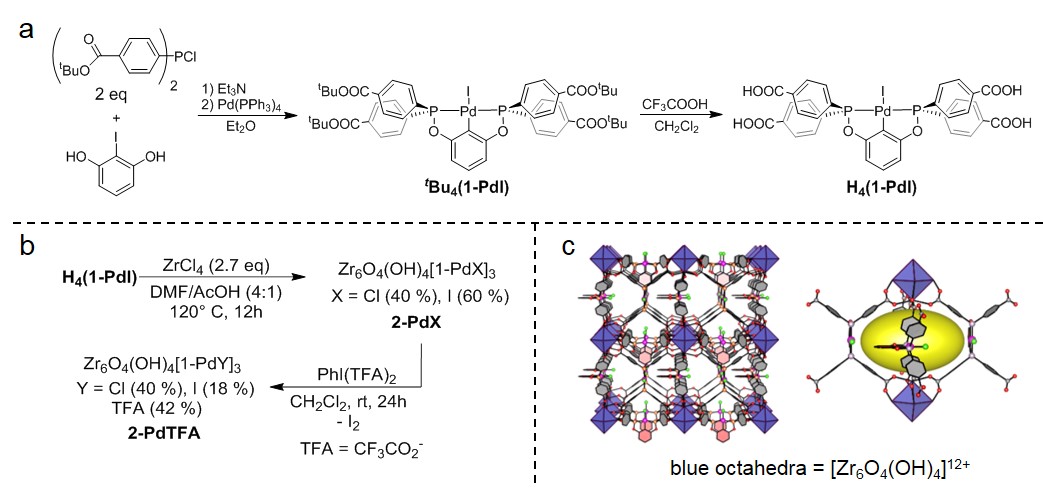
Figure 1. a) Synthesis of Pd-POCOP complex H4(1-PdI). b) Synthesis of 2-PdX and 2-PdTFA. c) Framework structure of 2-PdX.
Progress
Solvothermal reaction of the Pd-POCOP complex H4(1-PdI) with ZrCl4 generates Zr MOF 2-PdX (Figure 1). The structure of 2-PdX was determined from synchrotron powder X-ray diffraction (PXRD) data and corroborated by solid-state MAS 31P and 13C NMR spectroscopy. The mixed I–/Cl– occupancy of the Pd-bound halide was determined from elemental analysis and solid state NMR experiments. The MOF contains [Zr6O4(OH)4]12+ octahedra connected by [1-PdX]4- linkers that span each face of the cubic unit cell, generating ovoidal pores ~16 Å × 10 Å in diameter. 2-PdX is stable for weeks under ambient conditions and retains crystallinity upon exposure to strong acid (1 M HNO3) and base (0.1 M NaOH).
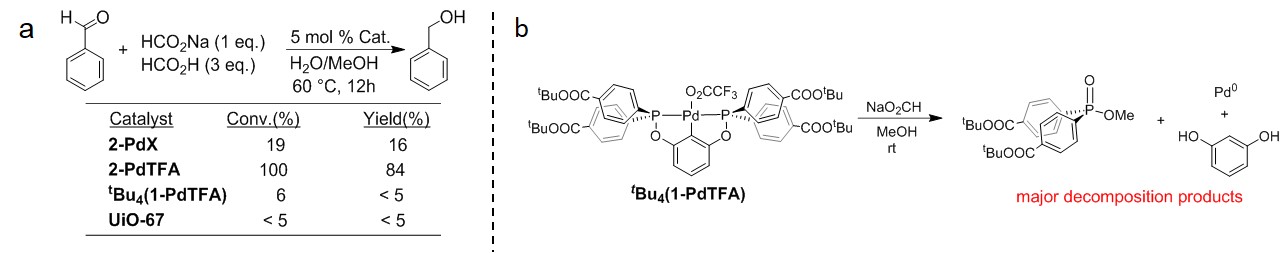
Figure 2. a) Transfer hydrogenation of benzaldehyde using MOF and homogeneous catalysts. b) Decomposition of homogeneous complex tBu4(1-PdTFA) upon treatment with NaO2CH.
2-PdX was screened as a catalyst for transfer hydrogenation of aldehydes using HCO2H/HCO2Na as a hydrogen source and found to afford low yields of the corresponding alcohol products (Figure 2a). We hypothesized that the poor leaving group ability of the I– ligands may prevent the ligand exchange necessary for catalysis. Consequently, a novel I-/TFA- (TFA- = CF3CO2-) ligand exchange procedure employing the hypervalent iodine reagent PhI(TFA)2 was used to generate 2-PdTFA, which proved to be more active for catalytic transfer hydrogenation of aldehydes. Hot filtration and Hg drop tests, along with the increased catalytic activity of 2-PdTFA relative to 2-PdX, support the immobilized pincer Pd centers as the active sites for catalysis. The homogeneous analogue, tBu4(1-PdTFA) exhibited little catalytic activity under the optimized conditions. Reaction of tBu4(1-PdTFA) with stoichiometric amounts of HCO2Na resulted in decomposition of the complex as shown in Figure 2b. The catalytic inefficacy and decomposition of tBu4(1-PdTFA) under these conditions demonstrate that rigid immobilization of the Pd-POCOP complex in the MOF 2-PdTFA greatly enhances stability and activity. These results have prompted us to continue exploring the synthesis and reactivity of MOFs assembled from other diphosphine pincer complexes.
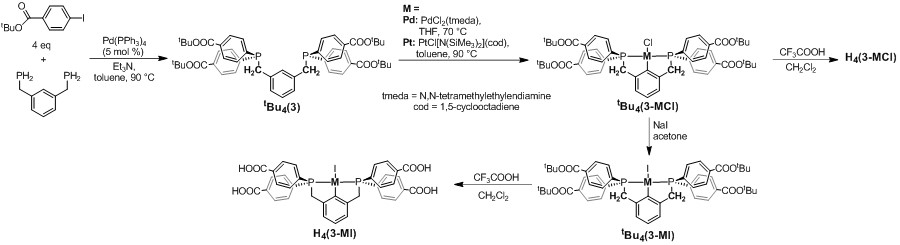
Figure 3. Synthesis of PCCCP complexes H4(3-MX) (M = Pd, Pt, X = Cl, I).
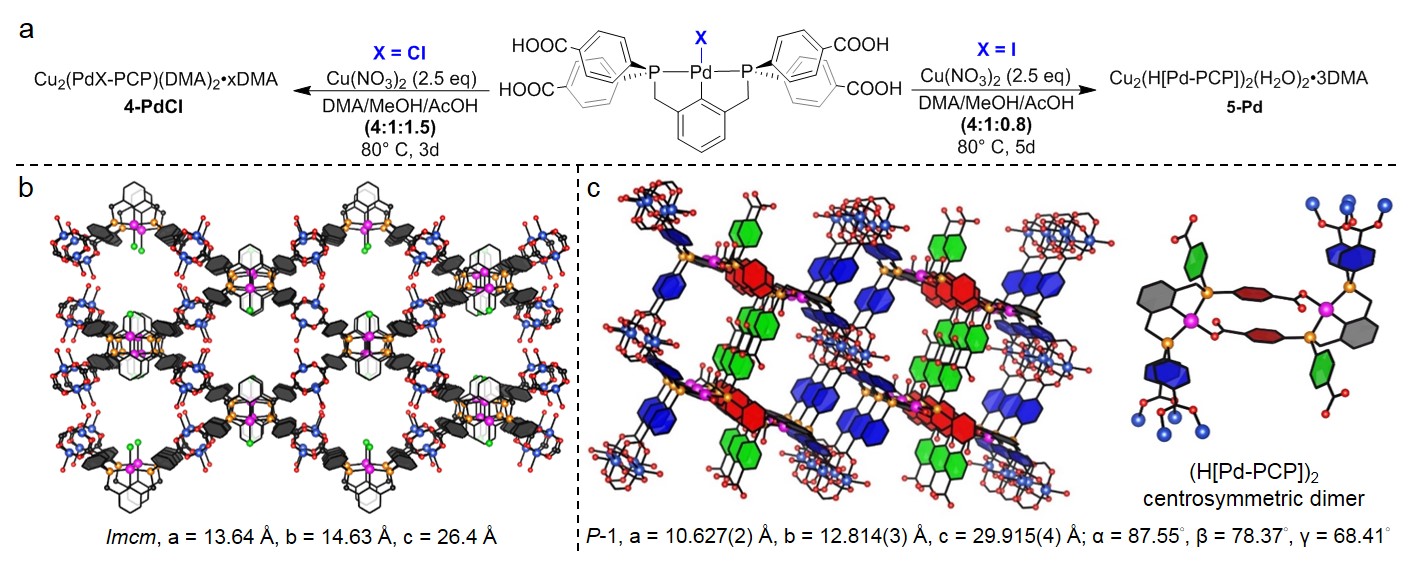
Figure 4. a) Synthesis of Cu MOFs 4-PdCl and 5-Pd. b) Structure of 4-PdCl. c) Structure of 5-Pd and dimerized Pd-PCP linker.
The PCCCP complexes H4(3-MX) (M = Pd, Pt, X = Cl, I) have been synthesized as shown in Figure 3. Two undergraduate researchers in my lab have obtained single crystals of two different Cu MOFs from solvothermal reactions of H4(3-PdCl) and H4(3-PdI) with Cu(NO3)2 (Figure 4a). The single crystal X-ray structure of 4-PdCl shows 2-dimensional zig-zag layers composed of [3-PdCl]4- linkers connected by Cu2(carboxylate)4 secondary building units (Figure 4b). The reaction of H4(3-PtCl) and Cu(NO3)2 under similar solvothermal conditions affords a microcrystalline powder (4-PtCl) with a powder X-ray diffraction pattern closely resembling that recorded for 4-PdCl. Single crystals of 5-Pd were obtained from solvothermal reaction of H4(3-PdI) with Cu(NO3)2 in a less acidic solvent mixture. The X-ray structure shows a 3-dimensional framework containing dimerized pincer complexes [H(3-PdI)]2 resulting from loss of HI and benzoate coordination to Pd (Figure 4c). The dimerized linkers are connected by Cu2(carboxylate)4 secondary building units, and two benzoic acid groups from each dimer decorate the small, solvent filled channels within the structure. These results illustrate the effects that small variations in the solvothermal reaction conditions and changes in the pincer complex structure can have on the MOF assembly process.
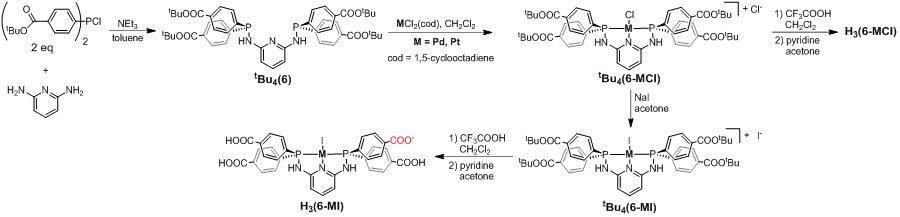
Figure 5. Synthesis of PNNNP complexes H3(6-MX) (M = Pd, Pt, X = Cl, I).
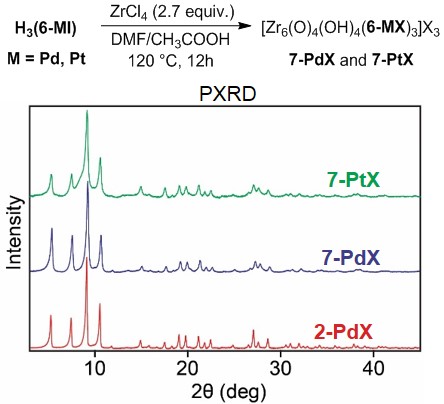
Figure 6. a) Synthesis of Zr MOFs 7-PdX and 7-PtX. b) Powder X-ray diffraction patterns of 2-PdX, 7-PdX and 7-PtX.
We have also begun to investigate MOF assembly with metallolinkers containing neutral PNP pincer ligands. The PNNNP complexes H3(6-MX) (M = Pd, Pt; X = Cl, I) have been synthesized as shown in Figure 5. Solvothermal reaction of H3(6-PdI) and H3(6-PtI) with ZrCl4 generates MOFs 7-PdX and 7-PtX, respectively. PXRD analysis indicates that these MOFs are isostructural to 2-PdX (Figure 6). Elemental analysis showed the presence of significant amounts of Cl and I in both frameworks, suggesting the possibility of mixed occupancy of the Pd/Pt-bound halide. Solvothermal reactions carried out using H3(6-PdCl) and H3(6-PtCl) generate amorphous solids, indicating that the identity of the coordinated halide plays a critical role in MOF assembly. Preliminary catalytic screening shows that 7-PdX and 7-PtX catalyze transfer hydrogenation of aldehydes and ketones under the reaction conditions shown in Figure 2a. Our current research is focused on determining the scope and efficiency of 7-PdX and 7-PtX as transfer hydrogenation catalysts as well as the assembly of MOFs containing other diphosphine pincer complexes.
Impact of Grant on PI’s Research Program
The funding provided by the ACS-PRF for this project has partially supported one postdoctoral research associate and one summer undergraduate researcher as well as provided research training for one graduate student and two undergraduate researchers. So far, the project has resulted in one high profile publication, and two manuscripts are in preparation. Preliminary results obtained from work sponsored by this award have been included in two proposals submitted to the National Science Foundation and Research Corporation for Science Advancement.