Reports: DNI755075-DNI7: The Living Anionic Polymerization of Functionalized Aziridines
Paul A. Rupar, PhD, University of Alabama
Background: Poly(ethylenimine) (PEI) is valuable for use in non-viral gene transfection, CO2 capture, and the formation of anti-microbial surfaces, among many other applications. Despite the resemblance of PEI to poly(ethylene oxide) (PEO), the polymerization of aziridine differs from that of ethylene oxide. Whereas ethylene oxide polymerizes anionically to form linear PEO, aziridine exclusively undergoes cationic ring-opening polymerization (CROP) to produce hyperbranched PEI (bPEI).
Linear PEI (lPEI) can be attained via the CROP of N-substituted aziridines, however control via these procedures has not been demonstrated. The standard route to lPEI is through the CROP of oxazolines to form poly(2-oxazoline)s (POx), followed by post-polymerization hydrolysis of Pox. The POx route achieves some control over molecular weight and polydispersity, but chain transfer reactions during the polymerization and difficulties in the hydrolysis to lPEI remain problematic
Research Progress: Our work is focused on developing the anionic ring-opening polymerization (AROP) of N-sulfonyl aziridines as a new route to linear PEI. A challenge encountered by us and others is that poly(N-sulfonylaziridines) suffer from poor solubility, thus making their synthesis problematic. We have overcome this difficulty by realizing that poly(N-sulfonylaziridine) random copolymers have greatly improved solubility compared to the homopolymers.
We recently published work showing that N-sulfonylaziridines can undergo AROP to form poly(N-sulfonylaziridines) which can subsequently be transformed into linear PEI. Using a 1:1 ratio of two different sulfonyl aziridines (MsAz and sBsAz, see Scheme 1), the resulting random copolymer P(MsAz-r-sBsAz) has greatly improved solubility and can be characterized and processed. For comparison, homopolymerization of MsAz or sBsAz results in only short chain oligomers that are insoluble in all common solvents. The basis for the improved solubility of P(MsAz-r-sBsAz) is likely due to the disruption of interchain packing caused by the random distribution of the two different monomers along the polymer backbone. Removal of the sulfonyl groups to create lPEI is performed using strong reducing agents
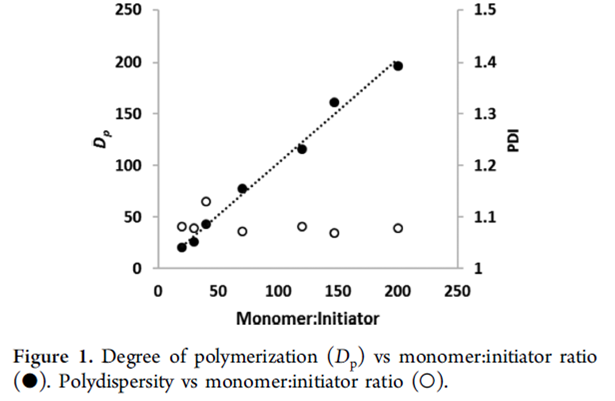
Since the copolymerization is a living AROP, we can target specific molecular weights by simply varying the ratio of the monomers to the anionic initiator (Figure 1). The polymers formed via this route have low molecular weight distributions with polydispersities typically less than 1.1. The polymerization is also amenable to the synthesis of block copolymers through sequential polymerizations.
Two graduate students in my group are supported by this grant: Canisius Pmbarushimana and Louis Reisman. Canisius is expected to graduate with a PhD in 2018 and Louis with a PhD in 2019. Both students are progressing well in their studies and their theses will be based on the research supported by this grant. The grant has also provided funds to travel to conferences to present this research.
Future Directions: In the second year of the project we are continuing the work on the polymerization of N-functionalized aziridines. A drawback to using sulfonyl aziridines to synthesize linear PEI, is that the removal of the sulfonyl aziridines requires harsh conditions. We will be examining alternative protecting groups that can still activate the aziridines to AROP, but which will be easier to remove postpolymerization.