Reports: ND955729-ND9: Strategies To Reduce Interfacial Viscosity in Nanoparticle-Based Enhanced Oil Recovery (EOR) Fluids
Surita R. Bhatia, PhD, State University of New York at Stony Brook
Nanoparticle and nanoparticle-polymer dispersions are emerging as promising candidates for fluids used in a number of Enhanced Oil Recovery (EOR) processes, including drilling and flooding. Rheological and interfacial properties of these fluids are known to be key to their use in EOR, and a great deal of fundamental research has been conducted on the bulk rheology and interfacial tension of nanoparticle-based dispersions. However, analysis of the flooding process suggests that an additional property, the interfacial viscosity, to be crucial in promoting oil recovery after displacement, with a low interfacial viscosity being desirable for EOR. Until recently, reliable measurement of interfacial rheology of nanoparticles at interfaces has been difficult. However, recent advances in instrumentation now allow for reproducible measurement of the rheology of nanoparticle-loaded interfaces.
Our goal for this project is to develop a fundamental understanding of how particle-particle interactions and particle-polymer interactions can be used to control the rheology of nanoparticles at air-water and air-brine interfaces. In doing so, we will test the extensibility of concepts from 3D (bulk) rheology of nanoparticles to 2D systems and will design new strategies to control the interfacial viscosity of nanoparticle dispersions. In Year 1 of the grant, our experiments have focused on the synthetic clay laponite, which is a disk-shaped nanoparticle with a diameter of 25 nm and a thickness of 0.92 nm with the empirical formula Na+0.7[(Si8Mg5.5Li0.3)O22(OH)4]-0.7, together with two water-soluble polymers, poly(ethylene oxide) (PEO) and poly(acrylic acid) (PAA) of varying molecular weights and concentrations.
Earlier studies by our group showed that the addition of PEO and PAA could be used to lower the bulk elastic modulus and complex viscosity of laponite dispersions through formation of particle clusters, induced by interparticle depletion attractions that arise from PEO and PAA chains. Over the past year, we have explored this phenomenon of cluster formation further and have investigated the extent to which these results could be translated to an interface; e.g., whether a similar mechanism may be used to create nanoparticle dispersions with a low interfacial viscosity through addition of polymer chains.
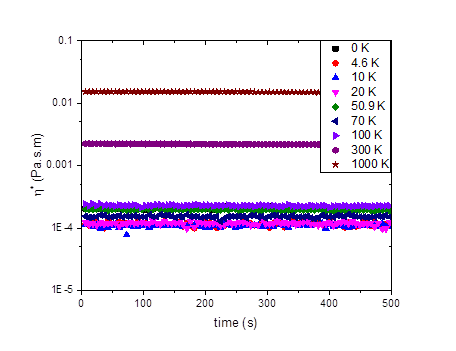
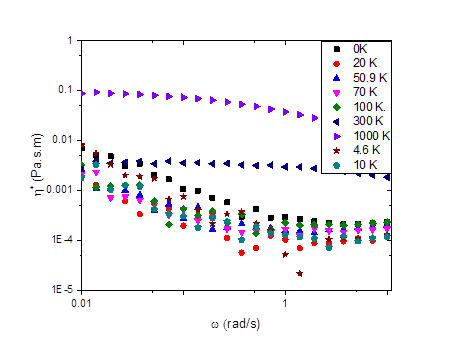
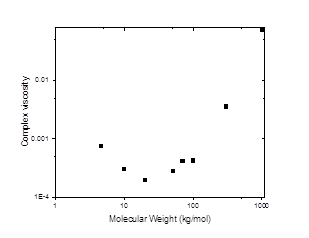
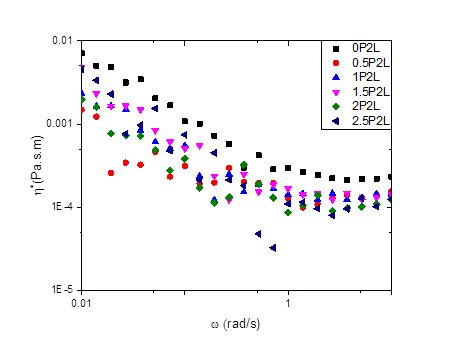
Figures 1-3 show our results to date for the complex interfacial viscosity for an oil-water interface with a dispersion containing 2.0 wt% laponite and 1.5 wt% PEO of varying molecular weights. PEO chains adsorb onto the surface of laponite particles, leading to complex impacts on the interparticle interactions including steric repulsion from adsorbed polymer chains, depletion attractions from free chains in solution, and bridging attractions from chains that adsorb on multiple particles. Although there is some scatter in the data, the lower molecular weight PEO chains do appear to decrease the viscosity of the interface, similar to the effects we observe in the bulk rheology of these systems. However, as the molecular weight of the chains increases, the complex interfacial viscosity begins to increase as well (Figure 3), possibly due to polymer bridging between particles.
Figure 1. The effects of various molecular weight of PEO on the complex viscosity of the interface of 1.5 wt% PEO and 2 wt% laponite at frequency of 1.0 Hz.
Figure 2. The effect of angular frequency on the complex viscosity of 1.5 wt% PEO and 2 wt% laponite.
Figure 3. The nonmonotonic dependence of complex viscosity on the molecular weight of PEO for 1.5 wt% PEO and 2 wt% laponite at frequency of 0.1 rad/s.
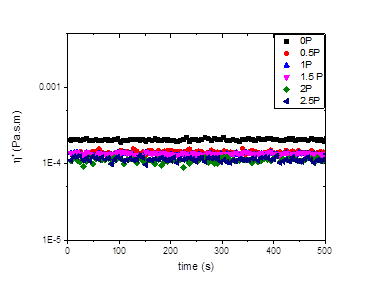
Poly(acrylic acid) chains do not adsorb onto laponite particles, and our results on the bulk rheology of similar systems suggests that these polymers have an even more dramatic effect on the rheology than PEO. Figures 4-5 should our preliminary results on the interfacial rheology of an oil-water interface with laponite and various concentrations of PAA chains with a molecular weight of 5,100 g/mol at the interface. These data also show some lowering of the viscosity, although the effects are not as dramatic as those seen in the laponite-PEO systems, and as above, there is some scatter in the data.
Figure 4. The effects of the concentration of 5.1 kg/mol PAA on the complex viscosity of 2 wt% laponite-oil interface at 1 Hz.
Figure 5. The effect of angular frequency on the complex viscosity of various concentrations of PAA and 2 wt% laponite.
Our preliminary results from Year 1 show promise for using this strategy to develop new fluids with a low interfacial viscosity for enhanced oil recovery applications. We are currently repeating both sets of experiments to verify reproducibility, and in Year 2 we plan to explore additional polymer molecular weights and concentrations and to investigate the mechanism for the decrease in viscosity that is observed.