Reports: DNI954904-DNI9: Probing Relationships between Carbon Nanostructure and Binary Molecular Mobility in "Crowded" Nanoporous Spaces
Ryan Lively, Georgia Institute of Technology
Statement of the Problem
Membrane-based separation technology is an attractive alternative to separation of petroleum-derived solvent molecules, as it is both more energy efficient and uses smaller physical footprint compared with other conventional separation techniques. Among the various possible membrane materials, polymers are the most widely applied due to their eminent processability. However, polymeric membranes suffer from aging, plasticization and compaction issues in harsh, solvent environments. Carbon molecular sieve (CMS) membranes are a promising alternative material that can potentially effectively perform challenging solvent separations. Indeed, CMS materials possess excellent thermal and chemical stability, even in aggressive, solvent environments. The separation properties of microporous CMS membranes are mainly decided by three factors: the choice of polymer precursor, the pyrolysis condition and the type of membrane geometry. To tailor microporous CMS membranes towards a particular structure capable of effective use in petroleum-derived solvent separations, a deeper understanding of CMS microstructure evolution is needed.
Experimental
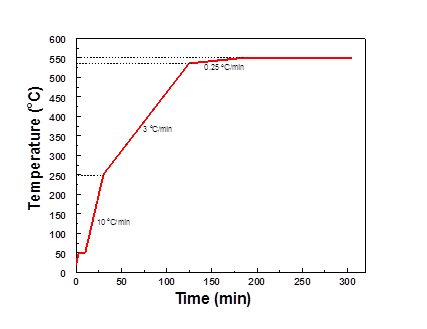
To create membrane solutions, 2 g of pre-dried polyvinylidene fluoride (PVDF, Alfa Aesar) were added into 8 g of dimethylacetamide (DMAc, 99%, Sigma-Aldrich). The polymer solutions were then mixed on a slowly-rotating shear mixer for 1-2 days and completely degassed before using. The dense (non-porous) PVDF films were cast on glass plates (MTI Corporation) with a doctor blade in a glove bag (Glascol) and allowed to slowly dry in a DMAc-saturated N2 atmosphere at room temperature for 1-2 days. The films were then dried at 80 oC overnight in a vacuum oven and crosslinked using a one-pot method. A cross-linking reaction solution was formulated by incorporating 2 g of sodium hydroxide, 15 g of methanol, 4 g of para-xylylenediamine and 2 g of magnesium oxide nanopowder (100 nm) into a mixture. The dried PVDF films were added into this well-mixed solution/dispersion and the cross-linking reaction was completed after 44 hours at room temperature on a shear mixer. Nitric acid solution [molarity?], DI-water, methanol and hexane were used to remove any residual byproducts and amine molecules. The crosslinked films were pyrolyzed in a quartz tube using a three-zone furnace (MTI corporation). The multi-step ramp rate for the pyrolysis protocol is shown in Figure 1. Similar pyrolysis protocols were used for different final pyrolysis temperature.
Figure 1, Pyrolysis protocol used in this project for a final pyrolysis temperature of 550 oC. The thermal soaking step was forest to 120 min at the final temperature.
Results and discussion
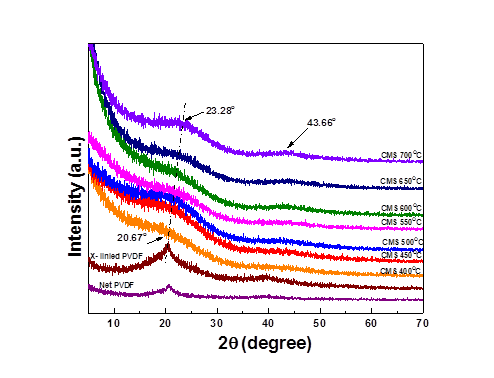
The structural characteristics of the CMS membranes derived from PVDF were determined by wide-angle X-ray diffraction (WAXD) patterns as a function of the pyrolysis temperature at 400, 450, 500, 550, 600, 650 and 700 oC. WAXD patterns in Figure 2 confirm the elimination of semi-crystallinity in the neat PVDF (and crosslinked PVDF) after pyrolysis and transformation into the less-ordered amorphous carbon structure. As shown in Fig.2, the observed broad reflections of the CMS membrane shifted incrementally from 20.67o to 23.28o with increasing pyrolysis temperature. The shift in reflections indicated that the interlayer spacing between neighboring planes decreased with increasing pyrolysis temperature. Based on Bragg’s law, the average d- spacing (comparative values of the degree of packing of the microporous carbon structure, interlayer distance) decrease from 4.76 Å to 4.23 Å depending on the extent of carbonization and atomic re-organization. The reduced average d spacing values of the carbon structures indicated the formation of an increased degree of packing of microporous carbon domains in the carbon matrices and lower free volume with increasing pyrolysis temperature. The reflections observed at 43.66 o (2.1 Å) were enhanced for the CMS membranes created at the higher pyrolysis temperatures. This reflection corresponds to the carbon-carbon spacing of the (100) plane in graphite, and suggests the formation of a more ordered (and likely non-porous) carbon structure.
Figure 2, XRD pattern for neat PVDF, crosslinked PVDF and CMS derived from crosslinked PVDF under different pyrolysis temperatures.
It is generally accepted that quantitative results of the sp3/sp2 ratio in nanocrytalline and amorphous carbon film can be obtained by nuclear magnetic resonance spectroscopy (NMR). The sp2 and sp3 carbon NMR spectra will show two separate peaks at different chemical shift positions, and the ratio of the sp3 to sp2 is equal to the peak integrated radio. Especially in cross polarization magic angle spinning (CPMAS) NMR spectra, sp3 13C show peaks from 0 to 100 ppm, however, sp2 13C peaks range from 100 to 200 ppm. Table 1 shows the sp2/sp3 carbon ratio for CMS derived from crosslinked PVDF under different pyrolysis temperature determined by CPMAS-NMR. As shown in table 1, the sp2/sp3 carbon ratio increased monotonously with the increase of pyrolysis temperature. Furthermore, no sp2/sp3 carbon ratio data were obtained for CMS under 650 and 700 oC by using CPMAS-NMR which is due to the high conductivity of samples. These results suggest that the CMS membranes under high pyrolysis temperature were more graphitic, which was similarly demonstrated by XRD patterns.
Table 1. SP2/SP3 carbon ratio for CMS derived from crosslinked PVDF under different pyrolysis temperature determined by CPMAS-NMR.
Pyrolysis temperature (oC) | SP2/SP3 carbon ratio |
400 | 1:0.6603 |
450 | 1:0.2002 |
500 | 1:0.2000 |
550 | 1:0.1320 |
600 | 1:0.0034 |
650 | - |
700 | - |
Conclusion
Carbon molecular sieve membranes derived from pyrolysis of polyvinylidene fluoride films were prepared at different final pyrolysis temperatures. Furthermore, the effects of pyrolysis temperature on the structure of the CMS were systematically studied by XRD and NMR. Increasing pyrolysis temperature led to decreasing interlayer spacing between neighboring planes and an increase of the sp2/sp3 carbon ratio in the CMS membranes. Additionally, the structure of the CMS becomes partially graphitic with increasing pyrolysis temperature. This research is anticipated to give a deeper understanding of microstructure evolution in CMS and provide guidelines to tailor the microporous structure of the films, which can be used to separate similarly-sized molecules found in petroleum-derived solvent.
Broader Impacts
The ACS PRF funding has been enabled the PI to train and mentor a female graduate student in his laboratory. The entirety of the ACS PRF funds have been used to support this Ph.D. student researcher doing the work described.