Reports: DNI353760-DNI3: Design of Homogeneous Catalysts for Upgrading Alcohols
Nathaniel Kolnik Szymczak, PhD, University of Michigan
This PRF grant targets mechanistic analyses to rationally optimize a series of transition-metal based complexes that mediate the oxidant-free catalytic dehydrogenation and dehydrogenative coupling of alcohols. During this reporting period, extensive progress has been made in two key areas: we have (1) developed the state-of-the-art process for upgrading ethanol to 1-butanol, and (2) delineated key mechanistic similarities and differences between alcohol and amine dehydrogenation. Summaries of both areas are highlighted below.
Upgrading Ethanol to 1-Butanol:
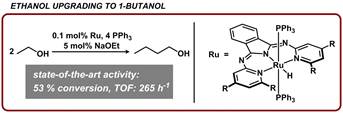
The insight that we gained during our mechanistic studies (years 1-2) was applied to upgrade a bio-relevant alcohol into a useful fuel additive. Ethanol is currently used as a gasoline additive. Compared to gasoline, the energy density of ethanol is 70%, and as a result, is only added in low blend ratios (~10%). Furthermore, storage of gasoline/ethanol mixtures is highly challenging because ethanol is miscible with water and is corrosive, and also separates from gasoline/ethanol blends. In contrast, 1-butanol has a much higher energy density (90% compared to gasoline) and is immiscible with water, mitigating corrosion and storage issues. Strategies for upgrading ethanol to higher order alcohols, such as 1-butanol, are thus highly desirable, yet limited. Our mechanistic studies uncovered a key design aspect that enabled ethanol to 1-butanol upgrading with high selectivity and an activity that exceeded all prior examples. Our system surpassed the activity of the previous systems by exhibiting TON of 530, with a TOF of 265 h-1 at 53% conversion using 0.1 mol% catalyst loading (or 700 h-1 for 10 ppm catalyst loading). This was the first report to show that ethanol can be upgraded to higher order alcohols with greater than 250 turnovers per hour and sets the stage for processes that use a bio-derived feedstock for one-step fuel-forming reactions. This work was highlighted in an international chemistry magazine (Chemistry World, January 13, 2016; https://www.chemistryworld.com/research/ethanol-to-butanol-conversion-shows-sustainable-potential/9335.article).
Figure 1. Catalytic upgrading of ethanol to 1-butanol using ruthenium N,N,N-pincer catalysts.
Dehydrogenation Mechanistic Studies:
In addition to catalyzing alcohol dehydrogenation and dehydrogenative coupling, several catalysts also promote a novel base-free and acceptorless double dehydrogenation of primary amines to nitriles with liberation of dihydrogen under moderate (< 120 °C) conditions. Nitriles are commonly prepared using toxic reagents, oxidative protocols, or require harsh conditions that are not tolerant of a broad range of functional groups and/or produce stoichiometric waste. Our catalytic dehydrogenation overcomes these limitations with high atom efficiency, and additionally produces a value-added product, H2. Although the reactivity is impressive, the origin and mechanism of this activity were unknown. We performed detailed mechanistic studies aimed at elucidating differences/similarities between alcohols and amines. These efforts were pursued in order to uncover key features that enable efficient catalysis, and provide further insight into the requirements/limitations of upgrading reactions that employ alcohol and/or amine feedstocks.
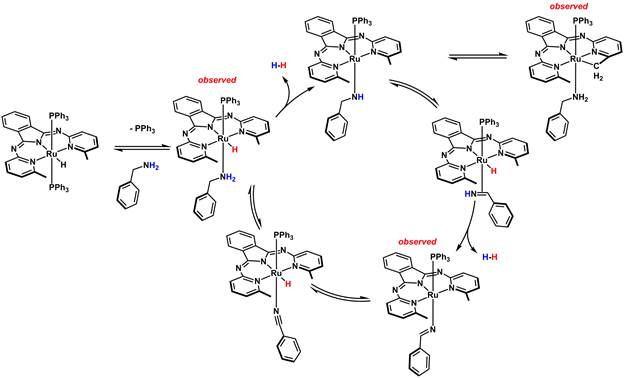
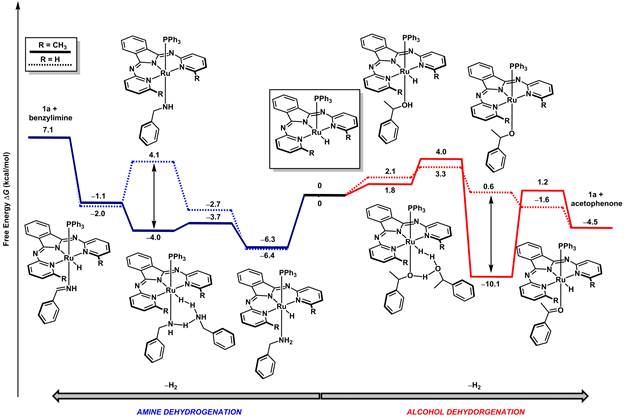
For amine dehydrogenation, most catalysts promote transamination, rather than double dehydrogenation. We employed a series of kinetic studies, stoichiometric reactions to probe catalytic intermediate species, prepared new catalyst variants, and also interrogated reaction pathways and intermediates with computational studies. We found that an inner-sphere, step-wise pathway is operative where proton transfer from coordinated substrate, is the turnover-limiting step. A unique feature of the catalyst is that a –CH group in an ortho- position of the pyridine ligands is required for catalysis; no catalysis is observed with a variant that lacks the CH (–CH3 or –iPr) groups. Importantly, through experimental and computational studies, we found that the appended CH3 units serve a critical role for substrate stabilization: a Ru-amido is a key intermediate that is stabilized by non-traditional CH---N non-covalent interactions. Furthermore, we addressed a larger issue in catalyst design, namely, how is the mechanistic pathway of a given reaction guided by the incorporation of cooperative units?
This was the first mechanistic analysis of acceptorless amine double dehydrogenation catalysis and importantly, we disclosed key similarities and differences between amines and the more commonly studied alcohols to provide the community with guiding principles for catalyst (re)design. We note that amine dehydrogenation represents an underexplored avenue for transfer hydrogenation reactivity, which typically is only examined using oxygen-containing H2 surrogates (alcohols, formic acid, etc). Our studies will find broad utility within many highly topical avenues of chemistry, including new ways that exploit hydrogen-borrowing strategies of amines and alcohols, requirements for dehydrogenation/coupling, catalyst (re)design, as well as chemoselective organic synthesis strategies.
Figure 2. Proposed mechanism of amine dehydrogenation using ruthenium N,N,N-pincer catalysts.
Figure 3. Free energy landscapes that compare the dehydrogenation of alcohols with amines using a single catalyst.
Summary of Support this Period:
The support provided by the ACS Petroleum Research Fund has helped to propel the PI’s research in the area of dehydrogenation and alcohol upgrading catalysis. We have applied a mechanistic approach to understand the catalytic mechanism, and we have used this information to develop more efficient catalysts. We applied these new catalysts to develop some of the best known catalysts that promote the ethanol upgrading reaction to 1-butanol. This reaction directly impacts the petroleum industry because 1-butanol is a drop-in fuel additive that can be obtained from renewable feedstocks, rather than fossil fuels. These efforts have received considerable attention from the chemical community, as noted by highlights in international chemistry magazines and also by the high citation counts of recent papers. In addition to the PI support, Tim Tseng and Lillian Hale are graduate students that have been supported by this grant and have made considerable progress. Tim Tseng has recently graduated and is now pursuing postdoctoral studies at the National Energy Technology Laboratory.